Recombinant Human TGF-beta 1 (Human Cell-expressed) Protein
Recombinant Human TGF-beta 1 (Human Cell-expressed) Protein Summary
Product Specifications
Ala279-Ser390
Analysis
Product Datasheets
7754-BH (with carrier)
7754-BH/CF (carrier free)
Carrier Free
CF stands for Carrier Free (CF). We typically add Bovine Serum Albumin (BSA) as a carrier protein to our recombinant proteins. Adding a carrier protein enhances protein stability, increases shelf-life, and allows the recombinant protein to be stored at a more dilute concentration. The carrier free version does not contain BSA.
In general, we advise purchasing the recombinant protein with BSA for use in cell or tissue culture, or as an ELISA standard. In contrast, the carrier free protein is recommended for applications, in which the presence of BSA could interfere.
7754-BH
| Formulation | Lyophilized from a 0.2 μm filtered solution in Acetonitrile and TFA with BSA as a carrier protein. *1 mg pack size (01M) is supplied as a 0.2 µm filtered solution in Acetonitrile and TFA with BSA as a carrier protein. |
| Reconstitution | Reconstitute at 100 μg/mL in sterile 4 mM HCl containing at least 0.1% human or bovine serum albumin. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage: | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
7754-BH/CF
| Formulation | Lyophilized from a 0.2 μm filtered solution in Acetonitrile and TFA. |
| Reconstitution | Reconstitute at 100 μg/mL in sterile 4 mM HCl. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage: | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Scientific Data
 View Larger
View Larger
Recombinant human TGF-beta 1 (7754-BH) inhibits recombinant mouse IL-4 induced proliferation in the HT-2 mouse T cell line. The ED50 for this effect is 0.04-0.2 ng/mL.
Background: TGF-beta 1
TGF‑ beta 1 (transforming growth factor beta 1) is one of three closely related mammalian members of the large TGF‑ beta superfamily that share a characteristic cystine knot structure (1‑7). TGF‑ beta 1, ‑2 and ‑3 are highly pleiotropic cytokines that are proposed to act as cellular switches that regulate processes such as immune function, proliferation and epithelial‑mesenchymal transition (1‑4). Each TGF‑ beta isoform has some non‑redundant functions; for TGF‑ beta 1, mice with targeted deletion show defects in hematopoiesis and endothelial differentiation, and die of overwhelming inflammation (2). Human TGF‑ beta 1 cDNA encodes a 390 amino acid (aa) precursor that contains a 29 aa signal peptide and a 361 aa proprotein (8). A furin-like convertase processes the proprotein to generate an N‑terminal 249 aa latency‑associated peptide (LAP) and a C‑terminal 112 aa mature TGF‑ beta 1 (8, 9). Disulfide-linked homodimers of LAP and TGF‑ beta 1 remain non‑covalently associated after secretion, forming the small latent TGF‑ beta 1 complex (8‑10). Covalent linkage of LAP to one of three latent TGF‑ beta binding proteins (LTBPs) creates a large latent complex that may interact with the extracellular matrix (9, 10). TGF‑ beta is activated from latency by pathways that include actions of the protease plasmin, matrix metalloproteases, thrombospondin 1 and a subset of integrins (10). Mature human TGF‑ beta 1 shares 100% aa identity with pig, dog and cow TGF‑ beta 1, and 99% aa identity with mouse, rat and horse TGF‑ beta 1. It demonstrates cross‑species activity (1). TGF‑ beta 1 signaling begins with high‑affinity binding to a type II ser/thr kinase receptor termed TGF‑ beta RII. This receptor then phosphorylates and activates a second ser/thr kinase receptor, TGF‑ beta RI (also called activin receptor‑like kinase (ALK) ‑5), or alternatively, ALK‑1. This complex phosphorylates and activates Smad proteins that regulate transcription (3, 11, 12). Contributions of the accessory receptors betaglycan (also known as TGF‑ beta RIII) and endoglin, or use of Smad-independent signaling pathways, allow for disparate actions observed in response to TGF‑ beta in different contexts (11).
- Derynck, R. and K. Miyazono (2008) Cold Spring Harbor Laboratory Press p. 29.
- Dunker, N. and K. Krieglstein (2000) Eur. J. Biochem. 267:6982.
- Wahl, S.M. (2006) Immunol. Rev. 213:213.
- Chang, H. et al. (2002) Endocr. Rev. 23:787.
- Lin, J.S. et al. (2006) Reproduction 132:179.
- Hinck, A.P. et al. (1996) Biochemistry 35:8517.
- Mittl, P.R.E. et al. (1996) Protein Sci. 5:1261.
- Derynck, R. et al. (1985) Nature 316:701.
- Miyazono, K. et al. (1988) J. Biol. Chem. 263:6407.
- Oklu, R. and R. Hesketh (2000) Biochem. J. 352:601.
- de Caestecker, M. et al. (2004) Cytokine Growth Factor Rev. 15:1.
- Zuniga, J.E. et al. (2005) J. Mol. Biol. 354:1052.
Citations for Recombinant Human TGF-beta 1 (Human Cell-expressed) Protein
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
74
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Unsupervised inter-domain transformation for virtually stained high-resolution mid-infrared photoacoustic microscopy using explainable deep learning
Authors: Park, E;Misra, S;Hwang, DG;Yoon, C;Ahn, J;Kim, D;Jang, J;Kim, C;
Nature communications
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
TGF? signaling sensitizes MEKi-resistant human melanoma to targeted therapy-induced apoptosis
Authors: Loos, B;Salas-Bastos, A;Nordin, A;Debbache, J;Stierli, S;Cheng, PF;Rufli, S;Wyss, C;Levesque, MP;Dummer, R;Wong, WW;Pascolo, S;Cantù, C;Sommer, L;
Cell death & disease
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
TGF?1-TNF? regulated secretion of neutrophil chemokines is independent of epithelial-mesenchymal transitions in breast tumor cells
Authors: SenGupta, S;Cohen, E;Serrenho, J;Ott, K;Coulombe, PA;Parent, CA;
bioRxiv : the preprint server for biology
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Tissue-specific functions of MSCs are linked to homeostatic muscle maintenance and alter with aging
Authors: Kurosawa, T;Ikemoto-Uezumi, M;Yoshimoto, Y;Minato, K;Kaji, N;Chaen, T;Hase, E;Minamikawa, T;Yasui, T;Horiguchi, K;Iino, S;Hori, M;Uezumi, A;
Aging cell
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Polymeric immunoglobulin receptor promotes Th2 immune response in the liver by increasing cholangiocytes derived IL-33: a diagnostic and therapeutic biomarker of biliary atresia
Authors: Li, Y;Li, TY;Qiao, Q;Zhang, MT;Tong, MX;Xu, LF;Zhang, ZB;
EBioMedicine
Species: Human
Sample Types: Whole Cells
Applications: Cell Culture -
IKK?-deficient macrophages impede cardiac repair after myocardial infarction by enhancing the macrophage-myofibroblast transition
Authors: Cho, HH;Rhee, S;Cho, DI;Jun, JH;Heo, H;Cho, SH;Kim, D;Wang, M;Kang, BG;Yoo, SJ;Cho, M;Lim, SY;Cho, JY;Jeong, IS;Kim, YS;Ahn, Y;
Experimental & molecular medicine
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
The Fibrotic Phenotype of Human Precision-Cut Lung Slices Is Maintained after Cryopreservation
Authors: Marimoutou, M;Patel, V;Kim, JH;Schaible, N;Alvarez, J;Hughes, J;Obermok, M;Rodríguez, CI;Kallarakal, T;Suki, B;Amin, K;Krishnan, R;Behrsing, HP;
Toxics
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
In vitro evidence for the potential of EGFR inhibitors to decrease the TGF-?1-induced dispersal of circulating tumour cell clusters mediated by EGFR overexpression
Authors: Hapeman, JD;Galwa, R;Carneiro, CS;Nedelcu, AM;
Scientific reports
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
TGF-?1 maintains the developmental potential of embryonic submandibular gland epithelia separated with mesenchyme
Authors: Li, H;Wang, G;Zhao, G;Liu, H;Liu, L;Cao, Y;Li, C;
Heliyon
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Interferon subverts an AHR-JUN axis to promote CXCL13+ T cells in lupus
Authors: Law, C;Wacleche, VS;Cao, Y;Pillai, A;Sowerby, J;Hancock, B;Horisberger, A;Bracero, S;Skidanova, V;Li, Z;Adejoorin, I;Dillon, E;Benque, IJ;Nunez, DP;Simmons, DP;Keegan, J;Chen, L;Baker, T;Brohawn, PZ;Al-Mossawi, H;Hao, LY;Jones, B;Rao, N;Qu, Y;Alves, SE;Accelerating Medicines Partnership: RA/SLE Network, ;Jonsson, AH;Shaw, KS;Vleugels, RA;Massarotti, E;Costenbader, KH;Brenner, MB;Lederer, JA;Hultquist, JF;Choi, J;Rao, DA;
Nature
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Identification of a distal enhancer regulating hedgehog interacting protein gene in human lung epithelial cells
Authors: Guo, F;Zhang, L;Yu, Y;Gong, L;Tao, S;Werder, RB;Mishra, S;Zhou, Y;Anamika, WJ;Lao, T;Inuzuka, H;Zhang, Y;Pham, B;Liu, T;Tufenkjian, TS;Richmond, BW;Wei, W;Mou, H;Wilson, AA;Hu, M;Chen, W;Zhou, X;
EBioMedicine
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Targeting HDAC6 to treat heart failure with preserved ejection fraction in mice
Authors: Ranjbarvaziri, S;Zeng, A;Wu, I;Greer-Short, A;Farshidfar, F;Budan, A;Xu, E;Shenwai, R;Kozubov, M;Li, C;Van Pell, M;Grafton, F;MacKay, CE;Song, X;Priest, JR;Argast, G;Mandegar, MA;Hoey, T;Yang, J;
Nature communications
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Epidermal growth factor receptor activation is essential for kidney fibrosis development
Authors: Cao, S;Pan, Y;Terker, AS;Arroyo Ornelas, JP;Wang, Y;Tang, J;Niu, A;Kar, SA;Jiang, M;Luo, W;Dong, X;Fan, X;Wang, S;Wilson, MH;Fogo, A;Zhang, MZ;Harris, RC;
Nature communications
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
TIM-3 Expression and M2 Polarization of Macrophages in the TGF?-Activated Tumor Microenvironment in Colorectal Cancer
Authors: Katagata, M;Okayama, H;Nakajima, S;Saito, K;Sato, T;Sakuma, M;Fukai, S;Endo, E;Sakamoto, W;Saito, M;Saze, Z;Momma, T;Mimura, K;Kono, K;
Cancers
Applications: Cell Culture -
A model for the dissemination of circulating tumour cell clusters involving platelet recruitment and a plastic switch between cooperative and individual behaviours
Authors: Hapeman, JD;Carneiro, CS;Nedelcu, AM;
BMC ecology and evolution
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Treprostinil Reconstitutes Mitochondrial Organisation and Structure in Idiopathic Pulmonary Fibrosis Cells
Authors: Fang, L;Chen, WC;Jaksch, P;Molino, A;Saglia, A;Roth, M;Lambers, C;
International journal of molecular sciences
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Senescence-Driven Inflammatory and Trophic Microenvironment Imprints Mesenchymal Stromal/Stem Cells in Osteoarthritic Patients
Authors: Fusi, G;Constantinides, M;Fissoun, C;Pichard, L;Pers, YM;Ferreira-Lopez, R;Pantesco, V;Poulet, C;Malaise, O;De Seny, D;Lemaitre, JM;Jorgensen, C;Brondello, JM;
Biomedicines
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Cancer-associated mesothelial cells are regulated by the anti-Müllerian hormone axis
Authors: Chauvin, M;Meinsohn, MC;Dasari, S;May, P;Iyer, S;Nguyen, NMP;Oliva, E;Lucchini, Z;Nagykery, N;Kashiwagi, A;Mishra, R;Maser, R;Wells, J;Bult, CJ;Mitra, AK;Donahoe, PK;Pépin, D;
Cell reports
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Organ function is preserved despite reorganization of niche architecture in the hair follicle
Authors: Wei, H;Du, S;Parksong, J;Pasolli, HA;Matte-Martone, C;Regot, S;Gonzalez, LE;Xin, T;Greco, V;
Cell stem cell
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Polymer-Functionalized Mitochondrial Transplantation to Fibroblasts Counteracts a Pro-Fibrotic Phenotype
Authors: Baudo, G;Wu, S;Massaro, M;Liu, H;Lee, H;Zhang, A;Hamilton, DJ;Blanco, E;
International journal of molecular sciences
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
TGF? signaling links early-life endocrine-disrupting chemicals exposure to suppression of nucleotide excision repair in rat myometrial stem cells
Authors: Bariani, MV;Cui, YH;Ali, M;Bai, T;Grimm, SL;Coarfa, C;Walker, CL;He, YY;Yang, Q;Al-Hendy, A;
Research square
Species: Rat
Sample Types: Whole Cells
Applications: Bioassay -
PI16+ reticular cells in human palatine tonsils govern T cell activity in distinct subepithelial niches
Authors: De Martin, A;Stanossek, Y;L�tge, M;Cadosch, N;Onder, L;Cheng, HW;Brandstadter, JD;Maillard, I;Stoeckli, SJ;Pikor, NB;Ludewig, B;
Nature immunology
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
TGF-beta is elevated in hyperuricemic individuals and mediates urate-induced hyperinflammatory phenotype in human mononuclear cells
Authors: V Klück, G Cab?u, L Mies, F Bukkems, L van Emst, R Bakker, A van Caam, HINT conso, TO Cri?an, LAB Joosten
Arthritis Research & Therapy, 2023-02-27;25(1):30.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Nsun2 coupling with RoRgammat shapes the fate of Th17 cells and promotes colitis
Authors: WL Yang, W Qiu, T Zhang, K Xu, ZJ Gu, Y Zhou, HJ Xu, ZZ Yang, B Shen, YL Zhao, Q Zhou, Y Yang, W Li, PY Yang, YG Yang
Nature Communications, 2023-02-16;14(1):863.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
5-FU-miR-15a Inhibits Activation of Pancreatic Stellate Cells by Reducing YAP1 and BCL-2 Levels In Vitro
Authors: VMD Almanzar, K Shah, JF LaComb, A Mojumdar, HR Patel, J Cheung, M Tang, J Ju, AB Bialkowska
International Journal of Molecular Sciences, 2023-02-16;24(4):.
Species: Human, Transgenic Mouse
Sample Types: Whole Cells
Applications: Bioassay -
ANGPTL4 stabilizes atherosclerotic plaques and modulates the phenotypic transition of vascular smooth muscle cells through KLF4 downregulation
Authors: DI Cho, MJ Ahn, HH Cho, M Cho, JH Jun, BG Kang, SY Lim, SJ Yoo, MR Kim, HS Kim, SJ Lee, LT Dat, C Lee, YS Kim, Y Ahn
Experimental & Molecular Medicine, 2023-02-13;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
DRAK2 contributes to type 1 diabetes by negatively regulating IL-2 sensitivity to alter regulatory T�cell development
Authors: AH Mandarano, TL Harris, BM Creasy, M Wehenkel, M Duggar, BA Wilander, A Mishra, JC Crawford, SA Mullen, KM Williams, M Pillai, AA High, MA McGargill
Cell Reports, 2023-02-10;42(2):112106.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
xcore: an R package for inference of gene expression regulators
Authors: M Migda?, T Arakawa, S Takizawa, M Furuno, H Suzuki, E Arner, CL Winata, B Kaczkowski
BMC Bioinformatics, 2023-01-11;24(1):14.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
TGF-beta signaling and Creb5 cooperatively regulate Fgf18 to control pharyngeal muscle development
Authors: J Feng, X Han, Y Yuan, CK Cho, E Jane?ková, T Guo, S Pareek, MS Rahman, B Zheng, J Bi, J Jing, M Zhang, J Xu, TV Ho, Y Chai
Elife, 2022-12-21;11(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Cell Culture -
Poricoic acid A suppresses renal fibroblast activation and interstitial fibrosis in UUO rats via upregulating Sirt3 and promoting beta-catenin K49 deacetylation
Authors: DQ Chen, L Chen, Y Guo, XQ Wu, TT Zhao, HL Zhao, HJ Zhang, MH Yan, GQ Zhang, P Li
Acta pharmacologica Sinica, 2022-12-05;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Nanoparticle endothelial delivery of PGC-1alpha attenuates hypoxia-induced pulmonary hypertension by attenuating EndoMT-caused vascular wall remodeling
Authors: D Cai, SY Chen
Redox Biology, 2022-10-28;58(0):102524.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Targeting hypoxia-induced tumor stemness by activating pathogen-induced stem cell niche defense.
Authors: Bhuyan S, Pal B, Pathak L, Saikia P, Mitra S, Gayan S, Mokhtari R, Li H, Ramana C, Baishya D, Das B
Front Immunol, 2022-09-29;13(0):933329.
Species: Complex Species Category
Sample Types: Whole Cells
Applications: Bioassay -
Airway basal cells show a dedifferentiated KRT17highPhenotype and promote fibrosis in idiopathic pulmonary fibrosis
Authors: B Jaeger, JC Schupp, L Plappert, O Terwolbeck, N Artysh, G Kayser, P Engelhard, TS Adams, R Zweigerdt, H Kempf, S Lienenklau, W Garrels, I Nazarenko, D Jonigk, M Wygrecka, D Klatt, A Schambach, N Kaminski, A Prasse
Nature Communications, 2022-09-26;13(1):5637.
Species: Human
Sample Types: Organoid
Applications: Bioassay -
LINC01146/F11R facilitates growth and metastasis of prostate cancer under the regulation of TGF-beta
Authors: X Guo, Y Gu, C Guo, L Pei, C Hao
The Journal of steroid biochemistry and molecular biology, 2022-09-23;0(0):106193.
Species: Human
Sample Types: Whole Cells
Applications: Cell Culture -
HDAC5-mediated Smad7 silencing through MEF2A is critical for fibroblast activation and hypertrophic scar formation
Authors: Y Gao, Y Liu, D Zheng, C Ho, D Wen, J Sun, L Huang, Y Liu, Q Li, Y Zhang
International journal of biological sciences, 2022-09-11;18(15):5724-5739.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
IL-17A in Human Liver: Significant Source of Inflammation and Trigger of Liver Fibrosis Initiation.
Authors: Kartasheva-Ebertz D, Gaston J, Lair-Mehiri L, Mottez E, Buivan T, Massault P, Scatton O, Gaujoux S, Vaillant J, Pol S, Lagaye S
Int J Mol Sci, 2022-08-29;23(17):.
Species: Human
Sample Types: Whole Tissue
Applications: Bioassay -
Kynurenine metabolites predict survival in pulmonary arterial hypertension: A role for IL-6/IL-6Ralpha
Authors: Z Cai, S Tian, T Klein, L Tu, LW Geenen, T Koudstaal, AE van den Bo, YB de Rijke, IKM Reiss, E Boersma, C van der Le, M Van Faasse, I Kema, DJ Duncker, KA Boomars, K Tran-Lundm, C Guignabert, D Merkus
Scientific Reports, 2022-07-19;12(1):12326.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
SMAD3 mutation in LDS3 causes bone fragility by impairing the TGF-beta pathway and enhancing osteoclastogenesis
Authors: A El-Gazzar, H Kang, N Fratzl-Zel, E Webb, AM Barnes, M Jovanovic, SG Mehta, V Datta, V Saraff, RK Dale, F Rauch, JC Marini, W Högler
Bone Reports, 2022-07-16;17(0):101603.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
CRISPR interference interrogation of COPD GWAS genes reveals the functional significance of desmoplakin in iPSC-derived alveolar epithelial cells
Authors: RB Werder, T Liu, KM Abo, J Lindstrom-, C Villacorta, J Huang, A Hinds, N Boyer, E Bullitt, M Liesa, EK Silverman, DN Kotton, MH Cho, X Zhou, AA Wilson
Oncogene, 2022-07-13;8(28):eabo6566.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Hypo-osmotic stress induces the epithelial alarmin IL-33 in the colonic barrier of ulcerative colitis
Authors: MD Gundersen, KB Larsen, KM Johnsen, R Goll, J Florholmen, G Haraldsen
Scientific Reports, 2022-07-07;12(1):11550.
Species: Human
Sample Types: Whole Tissue
Applications: Bioassay -
Sphingosine kinase 1 mediates sexual dimorphism in fibrosis in a mouse model of NASH.
Authors: Montefusco D, Jamil M, Maczis M, Schroeder W, Levi M, Ranjit S, Allegood J, Bandyopadhyay D, Retnam R, Spiegel S, Cowart L
Mol Metab, 2022-06-06;62(0):101523.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Modified Shenlingbaizhu Decoction represses the pluripotency of colorectal cancer stem cells by inhibiting TGF-beta mediated EMT program.
Authors: Dai Y, Wang H, Sun R, Diao J, Ma Y, Shao M, Xu Y, Zhang Q, Gao Z, Zeng Z, Zhang L, Sun X
Phytomedicine, 2022-06-01;103(0):154234.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Fibrotic Signaling in Cardiac Fibroblasts and Vascular Smooth Muscle Cells: The Dual Roles of Fibrosis in HFpEF and CAD
Authors: JC Bachmann, SJ Baumgart, AK Uryga, MH Bosteen, G Borghetti, M Nyberg, KM Herum
Cells, 2022-05-17;11(10):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Proximal tubular RAGE mediated the renal fibrosis in UUO model mice via upregulation of autophagy
Authors: B Liu, T Sun, H Li, S Qiu, Y Li, D Zhang
Cell Death & Disease, 2022-04-23;13(4):399.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Dedifferentiation of Human Cardiac Myofibroblasts Is Independent of Activation of COX-2/PGE2 Pathway
Authors: VT Luu, S Phan, ZQ Jin
International Journal of Molecular Sciences, 2022-03-11;23(6):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Identifying Function Determining Residues in Neuroimmune Semaphorin 4A
Authors: SP Chapoval, M Lee, A Lemmer, O Ajayi, X Qi, AF Neuwald, AD Keegan
International Journal of Molecular Sciences, 2022-03-11;23(6):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Down-regulation of the endothelial histone demethylase JMJD3 is associated with neointimal hyperplasia of arteriovenous fistulas in kidney failure
Authors: S Feng, EK Peden, Q Guo, TH Lee, Q Li, Y Yuan, C Chen, F Huang, J Cheng
The Journal of Biological Chemistry, 2022-03-10;0(0):101816.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
PD-L1 mediates lung fibroblast to myofibroblast transition through Smad3 and beta-catenin signaling pathways
Authors: X Guo, C Sunil, O Adeyanju, A Parker, S Huang, M Ikebe, TA Tucker, S Idell, G Qian
Scientific Reports, 2022-02-23;12(1):3053.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
PD-L1 promotes myofibroblastic activation of hepatic stellate cells by distinct mechanisms selective for TGF-beta receptor I versus II
Authors: L Sun, Y Wang, X Wang, A Navarro-Co, S Ilyas, N Jalan-Sakr, C Gan, X Tu, Y Shi, K Tu, Q Liu, Z Lou, H Dong, AH Sharpe, VH Shah, N Kang
Cell Reports, 2022-02-08;38(6):110349.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Quantifying Cell-Derived Changes in Collagen Synthesis, Alignment, and Mechanics in a 3D Connective Tissue Model.
Authors: Wilks B, Evans E, Howes A, Hopkins C, Nakhla M, Williams G, Morgan J
Adv Sci (Weinh), 2022-02-01;9(10):e2103939.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Bone marrow mesenchymal stem cell-derived exosomes improve renal fibrosis via regulating Smurf 2/Smad 7.
Authors: Liu Y, Guo W, Guo Y, Chen X, Liu W
Front Biosci (Landmark Ed), 2022-01-12;27(1):17.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Spatiotemporal control of myofibroblast activation in acoustically-responsive scaffolds via ultrasound-induced matrix stiffening.
Authors: Farrell E, Aliabouzar M, Quesada C, Baker B, Franceschi R, Putnam A, Fabiilli M
Acta Biomater, 2021-11-20;138(0):133-143.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Disease modeling of pulmonary fibrosis using human pluripotent stem cell-derived alveolar organoids
Authors: T Suezawa, S Kanagaki, K Moriguchi, A Masui, K Nakao, M Toyomoto, K Tamai, R Mikawa, T Hirai, K Murakami, M Hagiwara, S Gotoh
Stem Cell Reports, 2021-11-18;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Lobeglitazone, A Peroxisome Proliferator-Activated Receptor-Gamma Agonist, Inhibits Papillary Thyroid Cancer Cell Migration and Invasion by Suppressing p38 MAPK Signaling Pathway.
Authors: Jin J, Han J, Ha J, Baek H, Lim D
Endocrinol Metab (Seoul), 2021-10-14;36(5):1095-1110.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Monoacylglycerol lipase deficiency in the tumor microenvironment slows tumor growth in non-small cell lung cancer.
Authors: Kienzl M, Hasenoehrl C, Maitz K, Sarsembayeva A, Taschler U, Valadez-Cosmes P, Kindler O, Ristic D, Raftopoulou S, Santiso A, Barnthaler T, Brcic L, Hahnefeld L, Gurke R, Thomas D, Geisslinger G, Kargl J, Schicho R
Oncoimmunology, 2021-09-11;10(1):1965319.
Species: Human, Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Ventral stress fibers induce plasma membrane deformation in human fibroblasts.
Authors: Ghilardi S, Aronson M, Sgro A
Mol Biol Cell, 2021-06-30;32(18):1707-1723.
Species: Human
Sample Types: Transduced Whole Cells
Applications: Bioassay -
Application of a High-Content Screening Assay Utilizing Primary Human Lung Fibroblasts to Identify Antifibrotic Drugs for Rapid Repurposing in COVID-19 Patients.
Authors: Marwick J, Elliott R, Longden J, Makda A, Hirani N, Dhaliwal K, Dawson J, Carragher N
SLAS Discov, 2021-06-02;26(9):1091-1106.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
The Conversion of Human Tissue-Like Inflammatory Monocytes Into Macrophages.
Authors: Bsat M, Mehta H, Rubio M, Sarfati M
Curr Protoc, 2021-03-01;1(3):e74.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Identification of metabolism-associated genes and construction of a prognostic signature in bladder cancer
Authors: C Shen, J Liu, L Wang, Z Liang, H Niu, Y Wang
Cancer Cell International, 2020-11-04;20(1):538.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Tumor Cell-Derived TGFbeta1 Attenuates Antitumor Immune Activity of T Cells via Regulation of PD-1 mRNA.
Authors: Wu P, Geng B, Chen Q, Zhao E, Liu J, Sun C, Zha C, Shao Y, You B, Zhang W, Li L, Meng X, Cai J, Li X
Cancer Immunol Res, 2020-09-30;8(12):1470-1484.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Lack of evidence supporting a role of IFN-&beta and TGF-&beta in differential polarization of Bordetella pertussis specific-T cell responses
Authors: R da Silva A, LG Quiambao, F Soldevila, A Sutherland, B Peters, A Sette
Cytokine, 2020-09-29;137(0):155313.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
MAPK activity dynamics regulate non-cell autonomous effects of oncogene expression
Authors: TJ Aikin, AF Peterson, MJ Pokrass, HR Clark, S Regot
Elife, 2020-09-17;9(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Nucleolar protein NOP2/NSUN1 suppresses HIV-1 transcription and promotes viral latency by competing with Tat for TAR binding and methylation
Authors: W Kong, A Biswas, D Zhou, G Fiches, K Fujinaga, N Santoso, J Zhu
PLoS Pathog., 2020-03-16;16(3):e1008430.
Species: Human
Sample Types: Whole Cells
Applications: Cell Culture -
CD109 acts as a gatekeeper of the epithelial trait by suppressing epithelial to mesenchymal transition in squamous cell carcinoma cells in vitro
Authors: S Zhou, SD da Silva, PM Siegel, A Philip
Sci Rep, 2019-11-06;9(1):16317.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Accumulation of versican facilitates wound healing: Implication of its initial ADAMTS-cleavage site.
Authors: Islam S, Chuensirikulchai K, Khummuang S, Keratibumrungpong T, Kongtawelert P, Kasinrerk W, Hatano S, Nagamachi A, Honda H, Watanabe H
Matrix Biol, 2019-10-26;87(0):77-93.
Species: Transgenic Mouse
Sample Types: Whole Cells
Applications: Bioassay -
ERK Regulates HIF1alpha-Mediated Platinum Resistance by Directly Targeting PHD2 in Ovarian Cancer.
Authors: Li Z, Zhou W, Zhang Y, Sun W, Yung M, Sun J, Li J, Chen C, Li Z, Meng Y, Chai J, Zhou Y, Liu S, Cheung A, Ngan H, Chan D, Zheng W, Zhu W
Clin Cancer Res, 2019-07-08;25(19):5947-5960.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Ruxolitinib in combination with prednisone and nilotinib exhibit synergistic effects in human cells lines and primary cells from myeloproliferative neoplasms
Authors: A Arenas Cor, R Ayala Diaz, P Hernández-, J Gorrochate, D Primo, A Robles, ML Morales, J Ballestero, I Rapado, M Gallardo, M Linares, J Martínez-L
Haematologica, 2018-12-13;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Retinoic acid signaling is essential for airway smooth muscle homeostasis
Authors: F Chen, F Shao, A Hinds, S Yao, S Ram-Mohan, TA Norman, R Krishnan, A Fine
JCI Insight, 2018-08-23;3(16):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Normal breast-derived epithelial cells with luminal and intrinsic subtype-enriched gene expression document inter-individual differences in their differentiation cascade
Authors: B Kumar, MS Prasad, P Bhat-Naksh, M Anjanappa, M Kalra, N Marino, AMV Storniolo, X Rao, S Liu, J Wan, Y Liu, H Nakshatri
Cancer Res., 2018-07-11;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Posttranscriptional Regulation of LOXL1 Expression Via Alternative Splicing and Nonsense-Mediated mRNA Decay as an Adaptive Stress Response
Authors: D Berner, M Zenkel, F Pasutto, U Hoja, P Liravi, GC Gusek-Schn, FE Kruse, J Schödel, A Reis, U Schlötzer-
Invest. Ophthalmol. Vis. Sci., 2017-11-01;58(13):5930-5940.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
A Novel Bromodomain Inhibitor Reverses HIV-1 Latency through Specific Binding with BRD4 to Promote Tat and P-TEFb Association
Authors: H Huang, S Liu, M Jean, S Simpson, H Huang, M Merkley, T Hayashi, W Kong, I Rodríguez-, X Zhang, HO Yosief, H Miao, J Que, JJ Kobie, J Bradner, NG Santoso, W Zhang, J Zhu
Front Microbiol, 2017-06-07;8(0):1035.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
The human RNA surveillance factor Up-frameshift 1 inhibits hepatic cancer progression by targeting MRP2/ABCC2.
Authors: Zhang H, You Y, Zhu Z
Biomed Pharmacother, 2017-05-26;92(0):365-372.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Decoding NADPH oxidase 4 expression in human tumors
Authors: JL Meitzler, HR Makhlouf, S Antony, Y Wu, D Butcher, G Jiang, A Juhasz, J Lu, I Dahan, P Jansen-Dür, H Pircher, AM Shah, K Roy, JH Doroshow
Redox Biol, 2017-05-26;13(0):182-195.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Glucocorticoids Have Opposing Effects on Liver Fibrosis in Hepatic Stellate and Immune Cells.
Authors: Kim K, Lee J, Zhou Y, Harpavat S, Moore D
Mol Endocrinol, 2016-06-29;30(8):905-16.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay
FAQs
-
Will Recombinant Human TGF-beta 1 support the maintenance of either human or non-human cultured cells?
TGF-beta 1 performs multiple cellular functions, including cell growth, proliferation, differentiation and apoptosis. It would be necessary to determine the specific conditions for cell growth/proliferation for the cells being cultured.
-
Can Recombinant Human TGF beta 1 Protein (Catalog # 7754-BH or 7754-BH/CF) be reconstituted in PBS or water?
Recombinant Human TGF beta 1 Protein (Catalog # 7754-BH or 7754-BH/CF) needs to be reconstituted to 100 μg/mL in sterile 4 mM HCl containing 0.1% BSA or 4 mM HCl. This protein is hydrophobic by nature, so it requires an acidic buffer for reconstitution to go completely into solution. Stability testing has been evaluated on the protein with these reconstitution conditions; we can guarantee performance under these reconstitution conditions. When the protein is diluted with the cell culture media or buffers to the working concentration, the concentration of the acid should be buffered or negligible.
-
Has the half-life of recombinant human TGF beta 1 been determined?
While our Recombinant Human TGF-beta 1 Protein has been evaluated for bioactivity, the half-life of a specific protein will depend on experimental conditions, including the cell number, density, and media content. It is up to the end-user to determine the appropriate concentration and timing when adding Recombinant Human TGF-beta 1 Protein to individual experiments. For techniques and methodologies, we recommend reviewing our list of publications under the Citations tab on the product-specific web page to find reported use of our products in similar experimental layouts.
Reviews for Recombinant Human TGF-beta 1 (Human Cell-expressed) Protein
Average Rating: 4.8 (Based on 15 Reviews)
Have you used Recombinant Human TGF-beta 1 (Human Cell-expressed) Protein?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
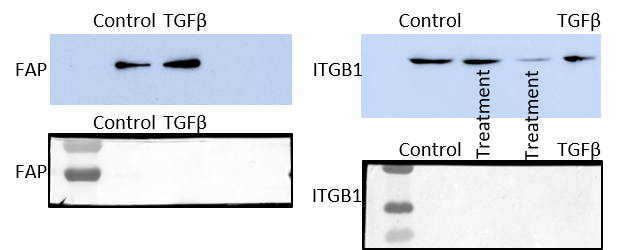
Reason for Rating: We used 10nM of TGF-beta to treat quiescent cancer associated fibroblasts, to transform them into activated cancer associated fibroblasts, as an positive control.
Reason for Rating: I used it in a number of cells in several assays and always worked as expected.
Reason for Rating: No discernible difference between HEK293-derived TGFb vs CHO-derived TGFb (cat#240-B) was found