Recombinant Human FGF basic/FGF2/bFGF (157 aa) Protein
Recombinant Human FGF basic/FGF2/bFGF (157 aa) Protein Summary
Product Specifications
Gly132-Ser288
Analysis
Product Datasheets
234-FSE (with carrier)
234-FSE/CF (carrier free)
Carrier Free
CF stands for Carrier Free (CF). We typically add Bovine Serum Albumin (BSA) as a carrier protein to our recombinant proteins. Adding a carrier protein enhances protein stability, increases shelf-life, and allows the recombinant protein to be stored at a more dilute concentration. The carrier free version does not contain BSA.
In general, we advise purchasing the recombinant protein with BSA for use in cell or tissue culture, or as an ELISA standard. In contrast, the carrier free protein is recommended for applications, in which the presence of BSA could interfere.
234-FSE
| Formulation | Lyophilized from a 0.2 μm filtered solution in Tris-HCl and NaCl with BSA as a carrier protein. |
| Reconstitution | Reconstitute at 100 μg/mL in sterile PBS containing at least 0.1% human or bovine serum albumin. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage: | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
234-FSE/CF
| Formulation | Lyophilized from a 0.2 μm filtered solution in Tris-HCl and NaCl. |
| Reconstitution | Reconstitute at 100 μg/mL in sterile PBS. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage: | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Scientific Data
 View Larger
View Larger
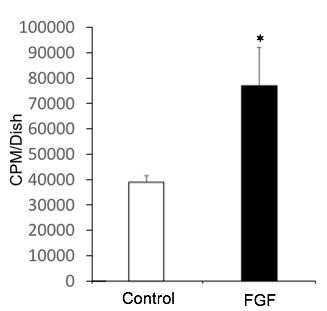
Recombinant Human FGF basic/FGF2/bFGF (157 aa) (Catalog # 234-FSE) stimulates cell proliferation of the NR6R‑3T3 mouse fibroblast cell line. The ED50 for this effect is 0.5-2.5 ng/mL.
 View Larger
View Larger
1 μg/lane of Recombinant Human FGF basic/FGF2/bFGF (157 aa) was resolved with SDS-PAGE under reducing (R) conditions and visualized by silver staining, showing a single band at 19 kDa.
Background: FGF basic/FGF2/bFGF
FGF basic is a member of the FGF family of at least 23 related mitogenic proteins which show 35-60% amino acid conservation. FGF acidic and basic, unlike the other members of the family, lack signal peptides and are apparently secreted by mechanisms other than the classical protein secretion pathway. FGF basic has been isolated from a number of sources, including neural tissue, pituitary, adrenal cortex, corpus luteum, and placenta. This factor contains four cysteine residues, but reduced FGF basic retains full biological activity, indicating that disulfide bonds are not required for this activity. A variety of forms of FGF basic are produced as a result of N-terminal extensions. These extensions affect localization of FGF basic in cellular compartments but do not affect biological activity. Binding of FGF to heparin or cell surface heparan sulfate proteoglycans is necessary for binding of FGF to high affinity FGF receptors. FGF acidic and basic appear to bind to the same high affinity receptors and show a similar range of biological activities. FGF basic stimulates the proliferation of all cells of mesodermal origin and many cells of neuroectodermal, ectodermal, and endodermal origin. FGF basic induces neuron differentiation, survival, and regeneration. FGF basic also modulates embryonic development and differentiation. These observed in vitro functions of FGF basic suggest FGF basic may play a role in vivo in the modulation of such normal processes as angiogenesis, wound healing and tissue repair, embryonic development and differentiation, and neuronal function and neural degeneration. Additionally, FGF basic may participate in the production of a variety of pathological conditions resulting from excessive cell proliferation and excessive angiogenesis.
- Coulier, F. et al. 1997, J. Mol. Evol. 44:43.
- Chen, C.H. et al. 2004, Curr. Vasc. Pharmacol. 2:33.
- Mohammadi, M. et al. 2005, Curr. Opin. Struct. Biol. 15:506.
- Fernig, D. et al. 1994, Prog. Growth Factor Res. 5:353.
Citations for Recombinant Human FGF basic/FGF2/bFGF (157 aa) Protein
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
14
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Betaglycan sustains HGF/Met signaling in lung cancer and endothelial cells promoting cell migration and tumor growth
Authors: Cervantes-Villagrana, RD;Mendoza, V;Hinck, CS;de la Fuente-León, RL;Hinck, AP;Reyes-Cruz, G;Vázquez-Prado, J;López-Casillas, F;
Heliyon
Species: Mouse, Porcine
Sample Types: Whole Cells
Applications: Bioassay -
The temporal balance between self-renewal and differentiation of human neural stem cells requires the amyloid precursor protein
Authors: Shabani, K;Pigeon, J;Benaissa Touil Zariouh, M;Liu, T;Saffarian, A;Komatsu, J;Liu, E;Danda, N;Becmeur-Lefebvre, M;Limame, R;Bohl, D;Parras, C;Hassan, BA;
Science advances
Species: Human
Sample Types: Organoids
Applications: Bioassay -
Organoids from mouse molar and incisor as new tools to study tooth-specific biology and development
Authors: F Hermans, L Hemeryck, C Bueds, M Torres Per, S Hasevoets, H Kobayashi, D Lambrechts, I Lambrichts, A Bronckaers, H Vankelecom
Stem Cell Reports, 2023-04-20;18(5):1166-1181.
Species: Mouse
Sample Types: Whole Cell
Applications: Bioassay -
Thymosin &beta4-Enhancing Therapeutic Efficacy of Human Adipose-Derived Stem Cells in Mouse Ischemic Hindlimb Model
Authors: JH Kim, IR Lim, CY Park, HJ Joo, JM Noh, SC Choi, SJ Hong, DS Lim
Int J Mol Sci, 2020-03-21;21(6):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Sorsby Fundus Dystrophy Mutation in Tissue Inhibitor of Metalloproteinase 3 (TIMP3) promotes Choroidal Neovascularization via a Fibroblast Growth Factor-dependent Mechanism
Authors: JH Qi, B Bell, R Singh, J Batoki, A Wolk, A Cutler, N Prayson, M Ali, H Stoehr, B Anand-Apte
Sci Rep, 2019-11-22;9(1):17429.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Anti-CD160, Alone or in Combination With Bevacizumab, Is a Potent Inhibitor of Ocular Neovascularization in Rabbit and Monkey Models
Authors: T Menguy, A Briaux, E Jeunesse, J Giustinian, A Calcei, T Guyon, J Mizrahi, H Haegel, V Duong, V Soler, P Brousset, A Bensussan, I Raymond Le, P Le Bouteil
Invest. Ophthalmol. Vis. Sci., 2018-06-01;59(7):2687-2698.
Species: Human
Sample Types:
Applications: In Vivo -
Ythdf2-mediated m6A mRNA clearance modulates neural development in mice
Authors: M Li, X Zhao, W Wang, H Shi, Q Pan, Z Lu, SP Perez, R Suganthan, C He, M Bjørås, A Klungland
Genome Biol., 2018-05-31;19(1):69.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
A Combination of Culture Conditions and Gene Expression Analysis Can Be Used to Investigate and Predict hES Cell Differentiation Potential towards Male Gonadal Cells.
Authors: Kjartansdottir K, Reda A, Panula S, Day K, Hultenby K, Soder O, Hovatta O, Stukenborg J
PLoS ONE, 2015-12-02;10(12):e0144029.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Antifibrotic and anti-inflammatory activity of the tyrosine kinase inhibitor nintedanib in experimental models of lung fibrosis.
Authors: Wollin L, Maillet I, Quesniaux V, Holweg A, Ryffel B
J Pharmacol Exp Ther, 2014-02-20;349(2):209-20.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Widespread potential for growth-factor-driven resistance to anticancer kinase inhibitors.
Authors: Wilson TR, Fridlyand J, Yan Y, Penuel E, Burton L, Chan E, Peng J, Lin E, Wang Y, Sosman J, Ribas A, Li J, Moffat J, Sutherlin DP, Koeppen H, Merchant M, Neve R, Settleman J
Nature, 2012-07-26;487(7408):505-9.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Interactions between important regulatory proteins and human alphaB crystallin.
Authors: Ghosh JG, Shenoy AK, Clark JI
Biochemistry, 2007-05-08;46(21):6308-17.
Species: Human
Sample Types: Peptide
Applications: ELISA-Based Protein Pin Array -
Neuritogenic activity of chondroitin/dermatan sulfate hybrid chains of embryonic pig brain and their mimicry from shark liver. Involvement of the pleiotrophin and hepatocyte growth factor signaling pathways.
Authors: Li F, Shetty AK, Sugahara K
J. Biol. Chem., 2006-12-04;282(5):2956-66.
Species: Fish - Prionace glauca (Blue Shark)
Sample Types: Peptide
Applications: Bioassay -
Placenta growth factor in diabetic wound healing: altered expression and therapeutic potential.
Authors: Cianfarani F, Zambruno G, Brogelli L, Sera F, Lacal PM, Pesce M, Capogrossi MC, Failla CM, Napolitano M, Odorisio T
Am. J. Pathol., 2006-10-01;169(4):1167-82.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Platelet-derived LIGHT induces inflammatory responses in endothelial cells and monocytes.
Authors: Otterdal K, Smith C, Oie E, Pedersen TM, Yndestad A, Stang E, Endresen K, Solum NO, Aukrust P, Damas JK
Blood, 2006-08-01;108(3):928-35.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay
FAQs
-
What receptors does FGF basic bind?
FGF receptor specificity has been reviewed in multiple citations. Please find more information at: //www.rndsystems.com/resources/articles/fibroblast-growth-factors-and-their-receptors
-
Does human FGF basic show activity on mouse cells?
Yes, it does. The bioassay uses NR-6 mouse fibroblast cells. There is 95% homology between the human and mouse protein and 98% homology between the human and mouse receptor.
Reviews for Recombinant Human FGF basic/FGF2/bFGF (157 aa) Protein
Average Rating: 5 (Based on 1 Review)
Have you used Recombinant Human FGF basic/FGF2/bFGF (157 aa) Protein?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by: