Normal Rabbit IgG Control Summary
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
Detection of Rabbit IgG Control by Flow Cytometry HepG2 human hepatocellular carcinoma cell line was stained with Rabbit Anti-Human Klotho beta Monoclonal Antibody (Catalog # MAB58891, filled histogram) or Rabbit IgG Isotype Control Antibody (Catalog # AB-105-C, open histogram), followed by Allophycocyanin-conjugated Anti-Rabbit IgG Secondary Antibody (Catalog # F0111). View our protocol for Staining Membrane-associated Proteins.
 View Larger
View Larger
Detection of Human IgG by Western Blot pSTAT5, but not NS1, interacts with the MCM complex.(A) Immunoprecipitation (IP) assay. Cell lysates of NS1Flag-expressing UT7/Epo-S1 cells were prepared for pull-down assays with either anti-Flag-conjugated beads or control beads. Immunoprecipitated proteins were examined for the presence of MCM2 by Western blotting. Blots were reprobed with rabbit anti-pSTAT5(Y694), anti-E2F5, and anti-Flag antibodies. Detection of E2F5 was used as a positive control for NS1 IP. (B) Co-IP assay. UT7/Epo-S1 cells were collected, washed, and lysed with RIPA buffer. After centrifugation, the supernatant was incubated with either rabbit anti-pSTAT5(Y694) or control IgG antibody. Immunoprecipitated proteins were blotted for the presence of the MCM complex with an anti-MCM5 antibody and for pSTAT5 with rabbit anti-pSTAT5(Y694). (C) Reverse Co-IP assay. Reverse Co-IP was performed with an anti-MCM5 antibody. Immunoprecipitated proteins were examined for pSTAT5, MCM2, and MCM5, respectively. (D) Co-IP of lysates treated with DNase. UT7/Epo-S1 cell lysates, either treated or untreated with DNase (750 units of Benzonase) were incubated with anti-pSTAT5(Y694) or control IgG antibodies for Co-IP assay, and immunoprecipitated proteins were examined for MCM2 by Western blot analysis. (E-H) Immunofluorescence analysis. (E&F) Mock- or B19V-infected CD36+ EPCs were co-stained with rabbit anti-STAT5 and mouse anti-MCM2 antibodies, followed by (E) incubation with respective secondary antibodies, or by (F) proximal ligation assay, which produces amplified signal for labeled molecules in close proximity. (G) CD36+ EPCs were incubated with either DMSO or pimozide (at 30 μM) for 2 days. And then the cells were co-stained with rabbit anti-STAT5 and mouse anti-MCM2 antibodies for proximity ligation assay. (H) Infected EPCs were stained with an anti-capsid antibody. Confocal images were taken with an Eclipse C1 Plus (Nikon) microscope at 100 × magnification. Image collected and cropped by CiteAb from the following open publication (https://dx.plos.org/10.1371/journal.ppat.1006370), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human IgG by Western Blot pSTAT5, but not NS1, interacts with the MCM complex.(A) Immunoprecipitation (IP) assay. Cell lysates of NS1Flag-expressing UT7/Epo-S1 cells were prepared for pull-down assays with either anti-Flag-conjugated beads or control beads. Immunoprecipitated proteins were examined for the presence of MCM2 by Western blotting. Blots were reprobed with rabbit anti-pSTAT5(Y694), anti-E2F5, and anti-Flag antibodies. Detection of E2F5 was used as a positive control for NS1 IP. (B) Co-IP assay. UT7/Epo-S1 cells were collected, washed, and lysed with RIPA buffer. After centrifugation, the supernatant was incubated with either rabbit anti-pSTAT5(Y694) or control IgG antibody. Immunoprecipitated proteins were blotted for the presence of the MCM complex with an anti-MCM5 antibody and for pSTAT5 with rabbit anti-pSTAT5(Y694). (C) Reverse Co-IP assay. Reverse Co-IP was performed with an anti-MCM5 antibody. Immunoprecipitated proteins were examined for pSTAT5, MCM2, and MCM5, respectively. (D) Co-IP of lysates treated with DNase. UT7/Epo-S1 cell lysates, either treated or untreated with DNase (750 units of Benzonase) were incubated with anti-pSTAT5(Y694) or control IgG antibodies for Co-IP assay, and immunoprecipitated proteins were examined for MCM2 by Western blot analysis. (E-H) Immunofluorescence analysis. (E&F) Mock- or B19V-infected CD36+ EPCs were co-stained with rabbit anti-STAT5 and mouse anti-MCM2 antibodies, followed by (E) incubation with respective secondary antibodies, or by (F) proximal ligation assay, which produces amplified signal for labeled molecules in close proximity. (G) CD36+ EPCs were incubated with either DMSO or pimozide (at 30 μM) for 2 days. And then the cells were co-stained with rabbit anti-STAT5 and mouse anti-MCM2 antibodies for proximity ligation assay. (H) Infected EPCs were stained with an anti-capsid antibody. Confocal images were taken with an Eclipse C1 Plus (Nikon) microscope at 100 × magnification. Image collected and cropped by CiteAb from the following open publication (https://dx.plos.org/10.1371/journal.ppat.1006370), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human IgG by Western Blot pSTAT5, but not NS1, interacts with the MCM complex.(A) Immunoprecipitation (IP) assay. Cell lysates of NS1Flag-expressing UT7/Epo-S1 cells were prepared for pull-down assays with either anti-Flag-conjugated beads or control beads. Immunoprecipitated proteins were examined for the presence of MCM2 by Western blotting. Blots were reprobed with rabbit anti-pSTAT5(Y694), anti-E2F5, and anti-Flag antibodies. Detection of E2F5 was used as a positive control for NS1 IP. (B) Co-IP assay. UT7/Epo-S1 cells were collected, washed, and lysed with RIPA buffer. After centrifugation, the supernatant was incubated with either rabbit anti-pSTAT5(Y694) or control IgG antibody. Immunoprecipitated proteins were blotted for the presence of the MCM complex with an anti-MCM5 antibody and for pSTAT5 with rabbit anti-pSTAT5(Y694). (C) Reverse Co-IP assay. Reverse Co-IP was performed with an anti-MCM5 antibody. Immunoprecipitated proteins were examined for pSTAT5, MCM2, and MCM5, respectively. (D) Co-IP of lysates treated with DNase. UT7/Epo-S1 cell lysates, either treated or untreated with DNase (750 units of Benzonase) were incubated with anti-pSTAT5(Y694) or control IgG antibodies for Co-IP assay, and immunoprecipitated proteins were examined for MCM2 by Western blot analysis. (E-H) Immunofluorescence analysis. (E&F) Mock- or B19V-infected CD36+ EPCs were co-stained with rabbit anti-STAT5 and mouse anti-MCM2 antibodies, followed by (E) incubation with respective secondary antibodies, or by (F) proximal ligation assay, which produces amplified signal for labeled molecules in close proximity. (G) CD36+ EPCs were incubated with either DMSO or pimozide (at 30 μM) for 2 days. And then the cells were co-stained with rabbit anti-STAT5 and mouse anti-MCM2 antibodies for proximity ligation assay. (H) Infected EPCs were stained with an anti-capsid antibody. Confocal images were taken with an Eclipse C1 Plus (Nikon) microscope at 100 × magnification. Image collected and cropped by CiteAb from the following open publication (https://dx.plos.org/10.1371/journal.ppat.1006370), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human Normal Rabbit IgG Control by Immunohistochemistry Elevated TGF-beta levels in the early inflammatory stage of AS. a Hematoxylin and eosin (H&E) staining and b Safranin O–Fast Green (SOFG) staining of normal and inflamed interspinous ligaments. In the AS group, the right panels show magnified views of the boxed area in the left panels. Scale bar: 100 μm (two panels on the right); 25 μm (left panel). c Immunostaining and d quantitative analysis of CD68-positive cells (brown) in the normal and inflamed interspinous ligaments (sagittal view). The bottom panels show magnified views of the boxed area in the top panels. Scale bar: 100 μm (top panels); 25 μm (bottom panels). e Immunostaining and f quantitative analysis of pSmad2/3-positive cells (brown) in the normal and inflamed interspinous ligaments (sagittal view) in normal ligaments and inflammatory ligaments. The bottom panels show magnified views of the boxed area in the top panels. Scale bar: 100 μm (top panels); 25 μm (bottom panels). g Immunostaining and h quantitative analysis of pSmad1/5/8-positive cells (brown) in the normal and inflamed interspinous ligaments (sagittal view). The bottom panels show magnified views of the boxed area in the top panels. Scale bar: 100 μm (top panels); 25 μm (bottom panels) Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/33731675), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human Normal Rabbit IgG Control by Immunohistochemistry Osteoclast resorption of bony interspinous ligaments releases active TGF-beta to drive the progression of ossification in AS patients. e Immunostaining and f quantitative analysis of CD68-positive cells (brown) in the normal and HO-formed interspinous ligaments (sagittal view). The bottom panels show magnified views of the boxed area in the top panels. Scale bar: 100 μm (top panel); 25 μm (bottom panel). Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/33731675), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human Normal Rabbit IgG Control by Immunohistochemistry Chondrocyte differentiation and cartilage formation in interspinous ligaments of AS patients before calcification. a Hematoxylin and eosin (H&E) staining and b Safranin O–Fast Green (SOFG) staining of normal and chondrogenic interspinous ligaments. In the AS group, the right panels show magnified views of the boxed area in the left panels. Scale bar: 100 μm (two panels on the right side); 25 μm (left panel). c Immunostaining and d quantitative analysis of collagen II-positive cells (brown) in the normal and chondrogenic interspinous ligaments (sagittal view). The bottom panels show a magnified view of the boxed area in the top panels. Scale bar: 100 μm (top panel); 25 μm (bottom panel). e Immunostaining and f quantitative analysis of pSmad2/3-positive cells (brown) in the normal and chondrogenic interspinous ligaments (sagittal view). The bottom panels show magnified views of the boxed area in the top panels. Scale bar: 100 μm (top panel); 25 μm (bottom panel). g Immunostaining and h quantitative analysis of pSmad1/5/8-positive cells (brown) in the normal and inflammatory interspinous ligaments (sagittal view). The bottom panels show magnified views of the boxed area in the top panels. Scale bar: 100 μm (top panels); 25 μm (bottom panels). C, cartilage; L, ligament Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/33731675), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human Normal Rabbit IgG Control by Immunohistochemistry Progression of endochondral heterotopic ossification in AS patients. i Immunostaining and j quantitative analysis of pSmad2/3-positive cells (brown) in the normal ligaments and endochondral-ossified interspinous ligaments (sagittal view). The bottom panels show magnified views of the boxed area in the top panels. Scale bar: 100 μm (top panel); 25 μm (bottom panel). k Immunostaining and l quantitative analysis of Osterix-positive cells (brown) in the normal and endochondral-ossified interspinous ligaments (sagittal view). The bottom panels show magnified views of the boxed area in the top panels. Scale bar: 100 μm (top panel); 25 μm (bottom panel). m Immunostaining and n quantitative analysis of pSmad1/5/8-positive cells (brown) in the normal and inflammatory interspinous ligaments (sagittal view). The bottom panels show magnified views of the boxed area in the top panels. Scale bar: 100 μm (top panels); 25 μm (bottom panels). B, bone; BM, bone marrow; C, cartilage Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/33731675), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human Normal Rabbit IgG Control by Immunohistochemistry Progression of endochondral heterotopic ossification in AS patients. g Immunostaining and h quantitative analysis of the number of CD68-positive osteoclast (brown) surface (OCS) per bone surface (BS). The bottom panels show magnified views of the boxed area in the top panels. Scale bar: 100 μm (top panel); 25 μm (bottom panel). i Immunostaining and j quantitative analysis of pSmad2/3-positive cells (brown) in the normal ligaments and endochondral-ossified interspinous ligaments (sagittal view). The bottom panels show magnified views of the boxed area in the top panels. Scale bar: 100 μm (top panel); 25 μm (bottom panel). k Immunostaining and l quantitative analysis of Osterix-positive cells (brown) in the normal and endochondral-ossified interspinous ligaments (sagittal view). The bottom panels show magnified views of the boxed area in the top panels. Scale bar: 100 μm (top panel); 25 μm (bottom panel). m Immunostaining and n quantitative analysis of pSmad1/5/8-positive cells (brown) in the normal and inflammatory interspinous ligaments (sagittal view). The bottom panels show magnified views of the boxed area in the top panels. Scale bar: 100 μm (top panels); 25 μm (bottom panels). B, bone; BM, bone marrow; C, cartilage Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/33731675), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human Normal Rabbit IgG Control by Immunohistochemistry Chondrocyte differentiation and cartilage formation in interspinous ligaments of AS patients before calcification. a Hematoxylin and eosin (H&E) staining and b Safranin O–Fast Green (SOFG) staining of normal and chondrogenic interspinous ligaments. In the AS group, the right panels show magnified views of the boxed area in the left panels. Scale bar: 100 μm (two panels on the right side); 25 μm (left panel). c Immunostaining and d quantitative analysis of collagen II-positive cells (brown) in the normal and chondrogenic interspinous ligaments (sagittal view). The bottom panels show a magnified view of the boxed area in the top panels. Scale bar: 100 μm (top panel); 25 μm (bottom panel). e Immunostaining and f quantitative analysis of pSmad2/3-positive cells (brown) in the normal and chondrogenic interspinous ligaments (sagittal view). The bottom panels show magnified views of the boxed area in the top panels. Scale bar: 100 μm (top panel); 25 μm (bottom panel). g Immunostaining and h quantitative analysis of pSmad1/5/8-positive cells (brown) in the normal and inflammatory interspinous ligaments (sagittal view). The bottom panels show magnified views of the boxed area in the top panels. Scale bar: 100 μm (top panels); 25 μm (bottom panels). C, cartilage; L, ligament Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/33731675), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human Normal Rabbit IgG Control by Immunohistochemistry Osteoclast resorption of bony interspinous ligaments releases active TGF-beta to drive the progression of ossification in AS patients. g Immunostaining and h quantitative analysis of pSmad2/3-positive cells in the normal and HO-formed interspinous ligaments (sagittal view). The bottom panels show magnified views of the boxed area in the top panels. Scale bar: 100 μm (top panel); 25 μm (bottom panel). i Immunostaining and j quantitative analysis of Osterix-positive cells (brown) in the normal and HO-formed interspinous ligaments (sagittal view). The bottom panels show magnified views of the boxed area in the top panels. Scale bar: 100 μm (top panel); 25 μm (bottom panel). k Immunostaining and l quantitative analysis of PDGF-BB-positive cells in the normal and HO-formed interspinous ligaments (sagittal view). The bottom panels show magnified views of the boxed area in the top panels. Scale bar: 100 μm (top panels); 25 μm (bottom panels). m CD31-positive (red) cells, n EMCN-positive (green) cells and quantification of the fold change of o CD31-positive vessels, p EMCN-positive vessels in the normal and HO-formed interspinous ligaments (sagittal view). q PGP9.5-positive (red) cells and CGRP-positive (green) cells and quantification of the fold change of r PGP9.5 and CGRP double positive nerves in the normal and HO-formed interspinous ligaments (sagittal view). s Immunostaining and t quantitative analysis of pSmad1/5/8-positive cells (brown) in the normal and inflammatory interspinous ligaments (sagittal view). The bottom panels show magnified views of the boxed area in the top panels. Scale bar: 100 μm (top panels); 25 μm (bottom panels) Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/33731675), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human Normal Rabbit IgG Control by Immunohistochemistry Progression of endochondral heterotopic ossification in AS patients. c Immunostaining and d quantitative analysis of collagen II-positive cells (brown) in the normal and chondrogenic interspinous ligaments (sagittal view). The bottom panels show magnified views of the boxed area in the top panels. Scale bar: 100 μm (top panels); 25 μm (bottom panels). Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/33731675), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human Normal Rabbit IgG Control by Immunohistochemistry Elevated TGF-beta levels in the early inflammatory stage of AS. a Hematoxylin and eosin (H&E) staining and b Safranin O–Fast Green (SOFG) staining of normal and inflamed interspinous ligaments. In the AS group, the right panels show magnified views of the boxed area in the left panels. Scale bar: 100 μm (two panels on the right); 25 μm (left panel). c Immunostaining and d quantitative analysis of CD68-positive cells (brown) in the normal and inflamed interspinous ligaments (sagittal view). The bottom panels show magnified views of the boxed area in the top panels. Scale bar: 100 μm (top panels); 25 μm (bottom panels). e Immunostaining and f quantitative analysis of pSmad2/3-positive cells (brown) in the normal and inflamed interspinous ligaments (sagittal view) in normal ligaments and inflammatory ligaments. The bottom panels show magnified views of the boxed area in the top panels. Scale bar: 100 μm (top panels); 25 μm (bottom panels). g Immunostaining and h quantitative analysis of pSmad1/5/8-positive cells (brown) in the normal and inflamed interspinous ligaments (sagittal view). The bottom panels show magnified views of the boxed area in the top panels. Scale bar: 100 μm (top panels); 25 μm (bottom panels) Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/33731675), licensed under a CC-BY license. Not internally tested by R&D Systems.
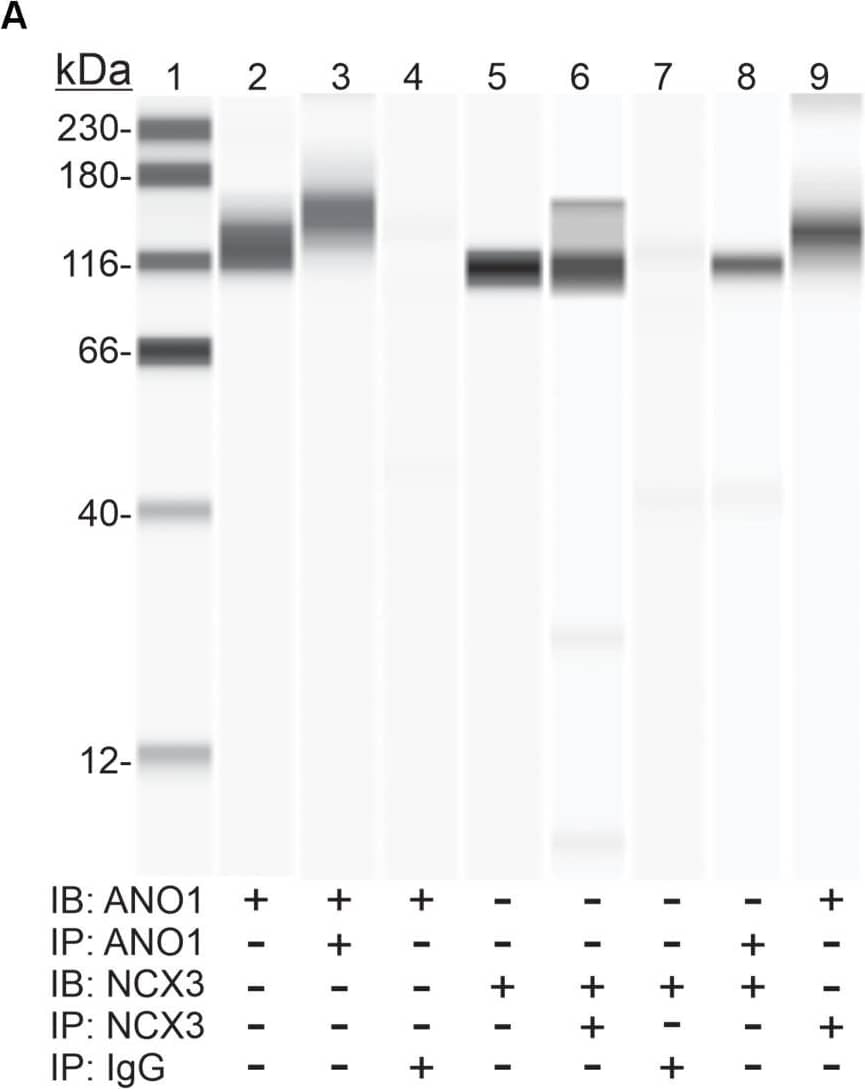 View Larger
View Larger
Detection of Mouse IgG by Western Blot Protein-protein interaction between ANO1 and NCX3. (A) Co- IP of ANO1 and NCX3 from small intestine smooth muscle membrane fraction. Immunoprecipitates (IP) were obtained by elution with low pH buffer, and immunoblotting (IB) was performed using Wes with anti-ANO1 and anti-NCX3 antibodies. Lanes: 1, protein standards; 2, ANO1 in membrane fraction (2.5 mg); 3, ANO1 in ANO1 immunoprecipitate (5 ml); 4, ANO1 in non-immune rabbit IgG IP; 5, NCX3 in membrane fraction (1 mg); 6, NCX3 in NCX3 immunoprecipitate (5 ml); 7, NCX3 in non-immune rabbit IgG IP; 8, NCX3 in Ano1 immunoprecipitate (5 ml); 9, ANO1 in NCX3 immunoprecipitate (5 ml) n = 4. (B) Association between ANO1 and NCX3 occurs in situ in ICC, as demonstrated by PLA. ICC from small intestine (green, left panel) were exposed to the rabbit anti-ANO1 and goat anti-NCX3 antibodies, followed by the Duolink minus-anti rabbit IgG and plus-anti goat IgG secondary antibodies and the ligation and amplification reactions. For the PLA negative control, ICC were exposed to rabbit anti-ANO1 and mouse anti-myosin regulatory light chain antibodies (upper left panel). PLA-positive red spots were detected by ANO1-NCX3 co-localization (lower left panel). Few red spots were observed in negative controls. Nuclear staining of ICC was achieved with DAPI (blue, left panel). Intact ICC were confirmed by differential interference contrast (DIC) (right panels). Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/32256387), licensed under a CC-BY license. Not internally tested by R&D Systems.
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: IgG
R&D Systems offers a range of secondary antibodies and controls for flow cytometry, immunohistochemistry, and Western blotting. We provide species-specific secondary antibodies that are available with a variety of conjugated labels.
Our NorthernLights fluorescent secondary antibodies are bright and resistant to photobleaching. We are currently offering secondary antibodies recognizing mouse, rat, goat, sheep, and rabbit IgG as well as chicken IgY. These reagents are available with three distinct excitation and emission maxima, making them ideal for multi-color fluorescence microscopy.
Product Datasheets
Citations for Normal Rabbit IgG Control
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
176
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Astaxanthin promotes M2 macrophages and attenuates cardiac remodeling after myocardial infarction by suppression inflammation in rats
Authors: Pan X, Zhang K, Shen C et al.
Chin. Med. J.
-
Kruppel-like factor 3 inhibition by mutated lncRNA Reg1cp results in human high bone mass syndrome
Authors: Yang M, Guo Q, Peng H et al.
J. Exp. Med.
-
Abnormal histone methylation is responsible for increased vascular endothelial growth factor 165a secretion from airway smooth muscle cells in asthma.
Authors: Clifford RL, John AE, Brightling CE
J. Immunol., 2012-06-11;189(2):819-31.
-
Peroxiredoxin 1 induces inflammatory cytokine response and predicts outcome of cardiogenic shock patients necessitating extracorporeal membrane oxygenation: an observational cohort study and translational approach.
Authors: Liu CH, Kuo SW, Hsu LM et al.
J Transl Med
-
Inhibitory input directs astrocyte morphogenesis through glial GABA B R
Authors: YT Cheng, E Luna-Figue, J Woo, HC Chen, ZF Lee, AS Harmanci, B Deneen
bioRxiv : the preprint server for biology, 2023-03-15;0(0):.
-
Mosquito Exosomal Tetraspanin CD151 Facilitates Flaviviral Transmission and Interacts with ZIKV and DENV2 Viral Proteins
Authors: Neupane, D;Bayzid, M;Neelakanta, G;Sultana, H;
International journal of molecular sciences
Species: Insect - Mosquito
Sample Types: Whole Cells
Applications: Neutralization -
Catalytic inhibition of KAT6/KAT7 enhances the efficacy and overcomes primary and acquired resistance to Menin inhibitors in MLL leukaemia
Authors: Gordon, SJV;Perner, F;MacPherson, L;Wenge, DV;Bourgeois, W;Fennell, K;Klaus, T;Petrovic, J;Horvath, J;Cao, J;Lapek, J;Uryu, S;White, J;Lam, EYN;Mu, XJ;Chan, YC;Gillespie, A;Blyth, B;Camerino, MA;Bozikis, YE;Holze, H;Knezevic, K;Balic, J;Stupple, PA;Street, IP;Monahan, BJ;Sharma, S;Wainwright, EN;Vassiliadis, D;Paul, TA;Armstrong, SA;Dawson, MA;
bioRxiv : the preprint server for biology
Species: Human
Sample Types: Whole Cells
Applications: Immunoprecipitation, Mass Spectrometry -
TGF-?1 maintains the developmental potential of embryonic submandibular gland epithelia separated with mesenchyme
Authors: Li, H;Wang, G;Zhao, G;Liu, H;Liu, L;Cao, Y;Li, C;
Heliyon
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
EGFR inhibits TNF-?-mediated pathway by phosphorylating TNFR1 at tyrosine 360 and 401
Authors: Nam, YW;Shin, JH;Kim, S;Hwang, CH;Lee, CS;Hwang, G;Kim, HR;Roe, JS;Song, J;
Cell death and differentiation
Species: Human
Sample Types: Cell Lysates
Applications: Immunoprecipitation Control -
The chemokine XCL1 functions as a pregnancy hormone to program offspring innate anxiety
Authors: Chen, RJ;Nabila, A;Gal Toth, J;Stuhlmann, H;Toth, M;
Brain, behavior, and immunity
Species: Mouse
Sample Types: In Vivo
Applications: In vivo assay -
Nuclear Glycoprotein A Repetitions Predominant (GARP) Is a Common Trait of Glioblastoma Stem-like Cells and Correlates with Poor Survival in Glioblastoma Patients
Authors: Zimmer, N;Trzeciak, ER;Müller, A;Licht, P;Sprang, B;Leukel, P;Mailänder, V;Sommer, C;Ringel, F;Tuettenberg, J;Kim, E;Tuettenberg, A;
Cancers
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
The deubiquitinase USP9X regulates RIT1 protein abundance and oncogenic phenotypes
Authors: Riley, AK;Grant, M;Snell, A;Vichas, A;Moorthi, S;Urisman, A;Castel, P;Wan, L;Berger, AH;
bioRxiv : the preprint server for biology
Species: Human
Sample Types: Cell Lysates
Applications: Immunoprecipitation -
CASC4/GOLM2 drives high grade serous carcinoma anoikis resistance through the recycling of EGFR
Authors: Bapat, J;Yamamoto, TM;Woodruff, ER;Qamar, L;Mikeska, RG;Aird, KM;Watson, ZL;Brubaker, LW;Bitler, BG;
Cancer gene therapy
Species: Human
Sample Types: Cell Lysates
Applications: Immunoprecipitation -
Spinal cord injury dysregulates fibro-adipogenic progenitors miRNAs signaling to promote neurogenic heterotopic ossifications
Authors: Jules Gueguen, Dorothée Girard, Bastien Rival, Juliette Fernandez, Marie-Emmanuelle Goriot, Sébastien Banzet
Commun Biol
-
SARS-CoV-2 ORF8 accessory protein is a virulence factor
Authors: Bello-Perez, M;Hurtado-Tamayo, J;Mykytyn, AZ;Lamers, MM;Requena-Platek, R;Schipper, D;Muñoz-Santos, D;Ripoll-Gómez, J;Esteban, A;Sánchez-Cordón, PJ;Enjuanes, L;Haagmans, BL;Sola, I;
mBio
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Characterization of a spontaneous mouse model of mild, accelerated aging via ECM degradation in emphysematous lungs
Authors: Tanino, R;Tsubata, Y;Hotta, T;Okimoto, T;Amano, Y;Takechi, M;Tanaka, T;Akita, T;Nagase, M;Yamashita, C;Wada, K;Isobe, T;
Scientific reports
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
The pro-inflammatory response to influenza A virus infection is fueled by endothelial cells
Authors: Lisa Bauer, Laurine C Rijsbergen, Lonneke Leijten, Feline FW Benavides, Danny Noack, Mart M Lamers et al.
Life Science Alliance
-
Spinal Cathepsin S promotes visceral hypersensitivity via FKN/CX3CR1/p38 MAPK signaling pathways
Authors: Sun, P;Lin, W;Weng, Y;Gong, J;Huang, Y;Tang, Y;Lin, C;Chen, A;Chen, Y;
Molecular pain
Species: Rat
Sample Types:
Applications: In Vivo -
Osteoclast-derived IGF1 induces RANKL production in osteocytes and contributes to Pagetic lesion formation
Authors: Miyagawa, K;Tenshin, H;Mulcrone, PL;Delgado-Calle, J;Subler, MA;Windle, JJ;Chirgwin, JM;Roodman, GD;Kurihara, N;
JCI insight
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
O-GlcNAcylation of RIPK1 rescues red blood cells from necroptosis
Authors: Junghwa Seo, Yeolhoe Kim, Suena Ji, Han Byeol Kim, Hyeryeon Jung, Eugene C. Yi et al.
Frontiers in Immunology
-
Elevated plasma complement factor H related 5 protein is associated with venous thromboembolism
Authors: Iglesias, MJ;Sanchez-Rivera, L;Ibrahim-Kosta, M;Naudin, C;Munsch, G;Goumidi, L;Farm, M;Smith, PM;Thibord, F;Kral-Pointner, JB;Hong, MG;Suchon, P;Germain, M;Schottmaier, W;Dusart, P;Boland, A;Kotol, D;Edfors, F;Koprulu, M;Pietzner, M;Langenberg, C;Damrauer, SM;Johnson, AD;Klarin, DM;Smith, NL;Smadja, DM;Holmström, M;Magnusson, M;Silveira, A;Uhlén, M;Renné, T;Martinez-Perez, A;Emmerich, J;Deleuze, JF;Antovic, J;Soria Fernandez, JM;Assinger, A;Schwenk, JM;Souto Andres, JC;Morange, PE;Butler, LM;Trégouët, DA;Odeberg, J;
Nature communications
Species: Human
Sample Types: Plasma
Applications: Mass Spectrometry -
Neutralization of excessive levels of active TGF-beta 1 reduces MSC recruitment and differentiation to mitigate peritendinous adhesion
Authors: YuSheng Li, Xiao Wang, Bo Hu, Qi Sun, Mei Wan, Andrew Carr et al.
Bone Research
-
BAFF and APRIL counterregulate susceptibility to inflammation-induced preterm birth
Authors: JR Doll, ME Moreno-Fer, TE Stankiewic, JL Wayland, A Wilburn, B Weinhaus, CA Chougnet, D Giordano, M Cappellett, P Presicce, SG Kallapur, N Salomonis, T Tilburgs, S Divanovic
Cell Reports, 2023-04-05;42(4):112352.
Species: Rabbit
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Aging, Plasminogen Activator Inhibitor 1, Brain Cell Senescence, and Alzheimer's Disease
Authors: Chun-Sun Jiang, Tapasi Rana, Lee-Way Jin, Susan A Farr, John E Morley, Hongwei Qin et al.
Aging Dis
-
Spontaneous Osteoclastogenesis, a risk factor for bone metastasis in advanced luminal A-type breast cancer patients
Authors: Valeria Fernández Vallone, Francisco Raúl Borzone, Leandro Marcelo Martinez, María Belén Giorello, Hosoon Choi, Federico Dimase et al.
Frontiers in Oncology
-
Anti-DNA-IgM Favors the Detection of NET-Associated Extracellular DNA
Authors: H Wang, AM Stehr, J Singh, L Zlatar, A Hartmann, K Evert, E Naschberge, S von Stillf, P Boor, LE Muñoz, J Knopf, M Stürzl, M Herrmann
International Journal of Molecular Sciences, 2023-02-17;24(4):.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Identification of AXL as a co-receptor for human parvovirus B19 infection of human erythroid progenitors
Authors: K Ning, W Zou, P Xu, F Cheng, EY Zhang, A Zhang-Chen, S Kleiboeker, J Qiu
Science Advances, 2023-01-11;9(2):eade0869.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization Control -
Identification and functional characterization of potential oncofetal targets in human hepatocellular carcinoma
Authors: Mei-Mei Li, Fan-En Kong, Guang-Meng Li, Yi-Ti He, Xiao-Feng Zhang, Cheng-Yang Zhang et al.
STAR Protocols
-
Neural crest cell-placodal neuron interactions are mediated by Cadherin-7 and N-cadherin during early chick trigeminal ganglion assembly
Authors: Caroline A. Halmi, Chyong-Yi Wu, Lisa A. Taneyhill
F1000Research
-
Adenosine A2a Receptor Antagonism Restores Additive Cytotoxicity by Cytotoxic T Cells in Metabolically Perturbed Tumors
Authors: Jeroen Slaats, Esther Wagena, Daan Smits, Annemarie A. Berends, Ella Peters, Gert-Jan Bakker et al.
Cancer Immunology Research
-
L‐type Voltage‐Gated calcium channels partly mediate Mechanotransduction in the intervertebral disc
Authors: Philip Poillot, Joseph W. Snuggs, Christine L. Le Maitre, Jacques M. Huyghe
JOR SPINE
-
The envelope protein of Zika virus interacts with apolipoprotein E early in the infectious cycle and this interaction is conserved on the secreted viral particles
Authors: Yannick Tréguier, Jade Cochard, Julien Burlaud-Gaillard, Roxane Lemoine, Philippe Chouteau, Philippe Roingeard et al.
Virology Journal
Species: Human
Sample Types: Microparticles
Applications: Neutralization Control -
Type I Interferon Receptor Subunit 1 Deletion Attenuates Experimental Abdominal Aortic Aneurysm Formation
Authors: T Shoji, J Guo, Y Ge, Y Li, G Li, T Ikezoe, W Wang, X Zheng, S Zhao, N Fujimura, J Huang, B Xu, RL Dalman
Biomolecules, 2022-10-21;12(10):.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Bone marrow and periosteal skeletal stem/progenitor cells make distinct contributions to bone maintenance and repair
Authors: EC Jeffery, TLA Mann, JA Pool, Z Zhao, SJ Morrison
Cell Stem Cell, 2022-10-21;29(11):1547-1561.e6.
Species: Mouse
Sample Types: Whole Cells
-
Distinct role of mitochondrial function and protein kinase C in intimal and medial calcification in vitro
Authors: Marina A. Heuschkel, Anne Babler, Jonas Heyn, Emiel P. C. van der Vorst, Marja Steenman, Maren Gesper et al.
Frontiers in Cardiovascular Medicine
-
In vitro and in vivo differences in neurovirulence between D614G, Delta And Omicron BA.1 SARS-CoV-2 variants
Authors: L Bauer, M Rissmann, FFW Benavides, L Leijten, P van Run, L Begeman, EJB Veldhuis K, B Lendemeije, H Smeenk, FMS de Vrij, SA Kushner, MPG Koopmans, B Rockx, D van Riel
Acta neuropathologica communications, 2022-09-05;10(1):124.
Species: Hamster
Sample Types: Whole Tissue
Applications: IHC Control -
Sox9 directs divergent epigenomic states in brain tumor subtypes
Authors: D Sardar, HC Chen, A Reyes, S Varadharaj, A Jain, C Mohila, R Curry, B Lozzi, K Rajendran, A Cervantes, K Yu, A Jalali, G Rao, SC Mack, B Deneen
Oncogene, 2022-07-15;119(29):e2202015119.
Species: Mouse
Sample Types: Tissue Homogenates
Applications: ChIP Control -
p63 silencing induces epigenetic modulation to enhance human cardiac fibroblast to cardiomyocyte-like differentiation
Authors: JP Pinnamanen, VP Singh, MB Kim, CT Ryan, A Pugazenthi, D Sanagasett, M Mathison, J Yang, TK Rosengart
Scientific Reports, 2022-07-06;12(1):11416.
Species: Mouse
Sample Types: Whole Cells
Applications: ChIP Control -
Stromal changes in the aged lung induce an emergence from melanoma dormancy
Authors: ME Fane, Y Chhabra, GM Alicea, DA Maranto, SM Douglass, MR Webster, VW Rebecca, GE Marino, F Almeida, BL Ecker, DJ Zabransky, L Hüser, T Beer, HY Tang, A Kossenkov, M Herlyn, DW Speicher, W Xu, X Xu, EM Jaffee, JA Aguirre-Gh, AT Weeraratna
Nature, 2022-06-01;606(7913):396-405.
Species: Mouse
Sample Types: In Vivo
Applications: Isotype Control -
Cross-talk between mutant p53 and p62/SQSTM1 augments cancer cell migration by promoting the degradation of cell adhesion proteins
Authors: S Mukherjee, M Maddalena, Y Lü, S Martinez, NB Nataraj, A Noronha, S Sinha, K Teng, V Cohen-Kapl, T Ziv, S Arandkar, O Hassin, R Chatterjee, AC Pirona, M Shreberk-S, A Gershoni, Y Aylon, Z Elazar, Y Yarden, D Schramek, M Oren
Proceedings of the National Academy of Sciences of the United States of America, 2022-04-19;119(17):e2119644119.
Species: Human
Sample Types: Cell Lysates
Applications: Immunoprecipitation -
DNA sequence-dependent formation of heterochromatin nanodomains
Authors: GJ Thorn, CT Clarkson, A Rademacher, H Mamayusupo, G Schotta, K Rippe, VB Teif
Nature Communications, 2022-04-06;13(1):1861.
Species: Mouse
Sample Types: Cell Lysates
Applications: ChIP -
Inhibition of Integrin alpha v beta 6 Activation of TGF‐ beta Attenuates Tendinopathy
Authors: Xiao Wang, Shen Liu, Tao Yu, Senbo An, Ruoxian Deng, Xiaohua Tan et al.
Advanced Science
-
Transcription Regulation of Tceal7 by the Triple Complex of Mef2c, Creb1 and Myod
Authors: Z Xiong, M Wang, S You, X Chen, J Lin, J Wu, X Shi
Biology, 2022-03-16;11(3):.
Species: Mouse
Sample Types: Whole Cells
Applications: ChIP -
ULK3-dependent activation of GLI1 promotes DNMT3A expression upon autophagy induction
Authors: P González-R, M Cheray, L Keane, P Engskog-Vl, B Joseph
Autophagy, 2022-02-28;0(0):1-12.
Species: Human
Sample Types: Cell Lysates
Applications: ChIP Control -
Macrophage IL-1beta promotes arteriogenesis by autocrine STAT3- and NF-kappaB-mediated transcription of pro-angiogenic VEGF-A
Authors: CS Mantsounga, C Lee, J Neverson, S Sharma, A Healy, JM Berus, C Parry, NM Ceneri, F López-Girá, HJ Chun, Q Lu, F Sellke, G Choudhary, AR Morrison
Cell Reports, 2022-02-01;38(5):110309.
Species: Human
Sample Types: Cell Lysates
Applications: Isotype Control -
C2cd6-encoded CatSper tau targets sperm calcium channel to Ca2+ signaling domains in the flagellar membrane
Authors: Jae Yeon Hwang, Huafeng Wang, Yonggang Lu, Masahito Ikawa, Jean-Ju Chung
Cell Reports
Species: Mouse, Transgenic Mouse
Sample Types: Tissue Homogenates
Applications: Bioassay -
Interplay in neural functions of cell adhesion molecule close homolog of L1 (CHL1) and Programmed Cell Death 6 (PDCD6)
Authors: Gabriele Loers, Thomas Theis, Helen Baixia Hao, Ralf Kleene, Sanjana Arsha, Nina Samuel et al.
FASEB BioAdvances
-
Hippo Pathway Effector Tead1 Induces Cardiac Fibroblast to Cardiomyocyte Reprogramming
Authors: Vivek P. Singh, Jaya P. Pinnamaneni, Aarthi Pugazenthi, Deepthi Sanagasetti, Megumi Mathison, James F. Martin et al.
Journal of the American Heart Association
Species: Rat
Sample Types: Whole Cells
Applications: ChIP (chromatin immunoprecipit -
Interactome analysis illustrates diverse gene regulatory processes associated with LIN28A in human iPS cell-derived neural progenitor cells
Authors: Nam-Kyung Yu, Daniel B. McClatchy, Jolene K. Diedrich, Sarah Romero, Jun-Hyeok Choi, Salvador Martínez-Bartolomé et al.
iScience
-
Pleiotropic Roles of NOTCH1 Signaling in the Loss of Maturational Arrest of Human Osteoarthritic Chondrocytes
Authors: M Minguzzi, V Panichi, S D'Adamo, S Cetrullo, L Cattini, F Flamigni, E Mariani, RM Borzì
International Journal of Molecular Sciences, 2021-11-05;22(21):.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
TGF beta 1 signaling protects chondrocytes against oxidative stress via FOXO1–autophagy axis
Authors: Ichiro Kurakazu, Yukio Akasaki, Hidetoshi Tsushima, Takuya Sueishi, Masakazu Toya, Masanari Kuwahara et al.
Osteoarthritis and Cartilage
-
Matricellular Protein SPARCL1 Regulates Blood Vessel Integrity and Antagonizes Inflammatory Bowel Disease
Authors: Daniela Regensburger, Clara Tenkerian, Victoria Pürzer, Benjamin Schmid, Thomas Wohlfahrt, Iris Stolzer et al.
Inflammatory Bowel Diseases
-
Phase separation of Epstein-Barr virus EBNA2 protein reorganizes chromatin topology for epigenetic regulation
Authors: Yiting Yang, Xidong Ye, Ranran Dai, Zhaoqiang Li, Yan Zhang, Wei Xue et al.
Communications Biology
-
N6-methyladenosine promotes induction of ADAR1-mediated A-to-I RNA editing to suppress aberrant antiviral innate immune responses
Authors: H Terajima, M Lu, L Zhang, Q Cui, Y Shi, J Li, C He
PloS Biology, 2021-07-29;19(7):e3001292.
Species: Human
Sample Types: Cell Lysate
Applications: RNA Immunoprecipitation Contro -
Site-specific O-GlcNAcylation of Psme3 maintains mouse stem cell pluripotency by impairing P-body homeostasis
Authors: F Pecori, N Kondo, C Ogura, T Miura, M Kume, Y Minamijima, K Yamamoto, S Nishihara
Cell Reports, 2021-07-13;36(2):109361.
Species: Human, Mouse
Sample Types: Cell Lysates
Applications: IP Control -
Extracellular vesicles carry SARS‐CoV‐2 spike protein and serve as decoys for neutralizing antibodies
Authors: Zach Troyer, Najwa Alhusaini, Caroline O. Tabler, Thomas Sweet, Karina Inacio Ladislau de Carvalho, Daniela M. Schlatzer et al.
Journal of Extracellular Vesicles
-
4-Hydroxy-2-nonenal antimicrobial toxicity is neutralized by an intracellular pathogen
Authors: Hannah Tabakh, Adelle P McFarland, Maureen K Thomason, Alex J Pollock, Rochelle C Glover, Shivam A Zaver et al.
eLife
-
Steroid hormones and human choriogonadotropin influence the distribution of alpha6-integrin and desmoplakin 1 in gland-like endometrial epithelial spheroids
Authors: V. U. Buck, M. T. Kohlen, A. K. Sternberg, B. Rösing, J. Neulen, R. E. Leube et al.
Histochemistry and Cell Biology
Species: Human
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Chondrogenesis mediates progression of ankylosing spondylitis through heterotopic ossification
Authors: Tao Yu, Jianguo Zhang, Wei Zhu, Xiao Wang, Yun Bai, Bin Feng et al.
Bone Research
-
Regulation of dual leucine zipper kinase activity through its interaction with calcineurin
Authors: JD Escobar, A Kutschenko, S Schröder, R Blume, KA Köster, C Painer, T Lemcke, W Maison, E Oetjen
Cellular Signalling, 2021-02-16;82(0):109953.
Species: Hamster
Sample Types: Cell Lysates
Applications: Immunoprecipitation Control -
Two distinct trophectoderm lineage stem cells from human pluripotent stem cells
Authors: A Mischler, V Karakis, J Mahinthaku, CK Carberry, A San Miguel, JE Rager, RC Fry, BM Rao
The Journal of Biological Chemistry, 2021-02-05;0(0):100386.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
CD48 Expression on Eosinophils in Nasal Polyps of Chronic Rhinosinusitis Patients
Authors: Yara Zoabi, Fidan Rahimli Alekberli, Yael Minai-Fleminger, Ron Eliashar, Francesca Levi-Schaffer
International Archives of Allergy and Immunology
-
Novel bi-allelic variants in DNAH2 cause severe asthenoteratozoospermia with multiple morphological abnormalities of the flagella
Authors: Y Gao, S Tian, Y Sha, X Zha, H Cheng, A Wang, C Liu, M Lv, X Ni, Q Li, H Wu, Q Tan, D Tang, B Song, D Ding, J Cong, Y Xu, P Zhou, Z Wei, Y Cao, Y Xu, F Zhang, X He
Reproductive biomedicine online, 2021-01-22;0(0):.
Species: Human
Sample Types: Spermatozoa
Applications: ICC -
Mutation of SPINOPHILIN (PPP1R9B) found in human tumors promotes the tumorigenic and stemness properties of cells
Authors: EM Verdugo-Si, AM Rojas, S Muñoz-Galv, D Otero-Albi, A Carnero
Theranostics, 2021-01-19;11(7):3452-3471.
Species: Human
Sample Types: Protein
Applications: Immunoprecipitation -
Mucoricin is a ricin-like toxin that is critical for the pathogenesis of mucormycosis
Authors: Sameh S. M. Soliman, Clara Baldin, Yiyou Gu, Shakti Singh, Teclegiorgis Gebremariam, Marc Swidergall et al.
Nature Microbiology
-
Genome-wide investigation of the dynamic changes of epigenome modifications after global DNA methylation editing
Authors: J Broche, G Kungulovsk, P Bashtrykov, P Rathert, A Jeltsch
Nucleic Acids Research, 2021-01-11;0(0):.
Species: Human
Sample Types: Cell Lysates
Applications: ChIP -
Molecular Targeting of H/MDM-2 Oncoprotein in Human Colon Cancer Cells and Stem-like Colonic Epithelial-derived Progenitor Cells
Authors: ANUSHA THADI, WILLIAM F. MORANO, MARIAN KHALILI, BLAKE D. BABCOCK, MOHAMMAD F. SHAIKH, DESHKA S. FOSTER et al.
Anticancer Research
-
Platelets Selectively Regulate the Release of BDNF, But Not That of Its Precursor Protein, proBDNF
Authors: Jessica Le Blanc, Samuel Fleury, Imane Boukhatem, Jean-Christophe Bélanger, Mélanie Welman, Marie Lordkipanidzé
Frontiers in Immunology
-
Mucin-type O-glycosylation controls pluripotency in mouse embryonic stem cells via Wnt receptor endocytosis
Authors: F Pecori, Y Akimoto, H Hanamatsu, JI Furukawa, Y Shinohara, Y Ikehara, S Nishihara
J. Cell. Sci., 2020-10-23;0(0):.
Species: Mouse
Sample Types: Cell Lysates
Applications: Immunoprecipitation -
Identification of drivers of breast cancer invasion by secretome analysis: insight into CTGF signaling
Authors: JW Hellinger, F Schömel, JV Buse, C Lenz, G Bauerschmi, G Emons, C Gründker
Sci Rep, 2020-10-21;10(1):17889.
Species: Human
Sample Types: Reference Standard
Applications: Control -
Identification of epilepsy-associated neuronal subtypes and gene expression underlying epileptogenesis
Authors: U Pfisterer, V Petukhov, S Demharter, J Meichsner, JJ Thompson, MY Batiuk, AA Martinez, NA Vasistha, A Thakur, J Mikkelsen, I Adorjan, LH Pinborg, TH Pers, J von Engelh, PV Kharchenko, K Khodosevic
Nat Commun, 2020-10-07;11(1):5038.
Species: Human
Sample Types: Tissue Homogenates
Applications: IHC -
Overexpression of microRNA-367 inhibits angiogenesis in ovarian cancer by downregulating the expression of LPA1
Authors: Q Zheng, X Dai, W Fang, Y Zheng, J Zhang, Y Liu, D Gu
Cancer Cell Int, 2020-10-02;20(0):476.
Species: Human, Mouse
Sample Types: Cell Lysates, Whole Tissue
Applications: IHC, Western Blot -
Donor HLA−DR Drives the Development of De Novo Autoimmunity Following Lung and Heart Transplantation
Authors: Ewa Jankowska−Gan, Vrushali V. Agashe, Diego A. Lema, Ying Zhou, Laura Gonzalez Bosc, Jeremy A. Sullivan et al.
Transplantation Direct
-
A role of the CTCF binding site at enhancer Ealpha in the dynamic chromatin organization of the Tcra-Tcrd locus
Authors: H Zhao, Z Li, Y Zhu, S Bian, Y Zhang, L Qin, AK Naik, J He, Z Zhang, MS Krangel, B Hao
Nucleic Acids Res., 2020-09-25;0(0):.
Species: Mouse
Sample Types: Cell Lysates
Applications: ChIP Control, Immunoprecipitation -
Parallel Murine and Human Plaque Proteomics Reveals Pathways of Plaque Rupture
Authors: Tomáš Vaisar, Jie H. Hu, Nathan Airhart, Kate Fox, Jay Heinecke, Roberto F. Nicosia et al.
Circulation Research
-
The DNA methyltransferase DNMT3A contributes to autophagy long-term memory
Authors: P González-R, M Cheray, J Füllgrabe, M Salli, P Engskog-Vl, L Keane, V Cunha, A Lupa, W Li, Q Ma, K Dreij, MG Rosenfeld, B Joseph
Autophagy, 2020-09-14;0(0):1-19.
Species: Human
Sample Types: Cell Lysates
Applications: Immunoprecipitation -
Cholesterol crystals use complement to increase NLRP3 signaling pathways in coronary and carotid atherosclerosis
Authors: N Niyonzima, SS Bakke, I Gregersen, S Holm, Ø Sandanger, HL Orrem, B Sporsheim, L Ryan, XY Kong, TB Dahl, M Skjelland, KK Sørensen, AM Rokstad, A Yndestad, E Latz, L Gullestad, GØ Andersen, JK Damås, P Aukrust, TE Mollnes, B Halvorsen, T Espevik
EBioMedicine, 2020-09-11;60(0):102985.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Lipid-specific IgMs induce antiviral responses in the CNS: implications for progressive multifocal leukoencephalopathy in multiple sclerosis
Authors: L Hayden, T Semenoff, V Schultz, SF Merz, KJ Chapple, M Rodriguez, AE Warrington, X Shi, CS McKimmie, JM Edgar, K Thümmler, C Linington, M Pingen
Acta Neuropathol Commun, 2020-08-13;8(1):135.
Species: Mouse, Rat
Sample Types: Whole Cells
Applications: Isotype Control, Neutralization Control -
Leptospiral LPS escapes mouse TLR4 internalization and TRIF?associated antimicrobial responses through O antigen and associated lipoproteins
Authors: D Bonhomme, I Santecchia, F Vernel-Pau, M Caroff, P Germon, G Murray, B Adler, IG Boneca, C Werts
PLoS Pathog., 2020-08-13;16(8):e1008639.
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry Control -
Abnormal Microvascular Architecture, Fibrosis, and Pericyte Characteristics in the Calf Muscle of Peripheral Artery Disease Patients with Claudication and Critical Limb Ischemia
Authors: CJ Mietus, TJ Lackner, PS Karvelis, GT Willcockso, CM Shields, NG Lambert, P Koutakis, MA Fuglestad, H Hernandez, GR Haynatzki, JKS Kim, HK DeSpiegela, II Pipinos, GP Casale
J Clin Med, 2020-08-08;9(8):.
Species: Bovine, Human
Sample Types: Whole Cells, Whole Tissue
Applications: ICC, Isotype Control -
Neonatal exposure to chlordecone alters female social behaviors and central estrogen alpha receptor expression in socially monogamous mandarin voles
Authors: Ting Lian, Xudong Zhang, Xiye Wang, Rong Wang, Huan Gao, Fadao Tai et al.
Toxicology Research
-
Regulation of Spermine Oxidase through Hypoxia-Inducible Factor-1alpha Signaling in Retinal Glial Cells under Hypoxic Conditions
Authors: D Wu, K Noda, M Murata, Y Liu, A Kanda, S Ishida
Invest. Ophthalmol. Vis. Sci., 2020-06-03;61(6):52.
Species: Human
Sample Types: Whole Tissue
Applications: Isotype Control -
Entry and exit of chemotherapeutically-promoted cellular dormancy in glioblastoma cells is differentially affected by the chemokines CXCL12, CXCL16, and CX3CL1
Authors: Vivian Adamski, Kirsten Hattermann, Carolin Kubelt, Gesa Cohrs, Ralph Lucius, Michael Synowitz et al.
Oncogene
-
Na+/Ca2 + Exchange and Pacemaker Activity of Interstitial Cells of Cajal
Authors: Haifeng Zheng, Bernard T. Drumm, Mei Hong Zhu, Yeming Xie, Kate E. O’Driscoll, Salah A. Baker et al.
Frontiers in Physiology
-
Complement inhibitor factor H expressed by breast cancer cells differentiates CD14+ human monocytes into immunosuppressive macrophages
Authors: Karolina I. Smolag, Christine M. Mueni, Karin Leandersson, Karin Jirström, Catharina Hagerling, Matthias Mörgelin et al.
OncoImmunology
-
Ophiopogonin D promotes bone regeneration by stimulating CD31hi EMCNhi vessel formation
Authors: Mi Yang, Chang‐Jun Li, Ye Xiao, Qi Guo, Yan Huang, Tian Su et al.
Cell Proliferation
-
Roxatidine inhibits fibrosis by inhibiting NF?kappaB and MAPK signaling in macrophages sensing breast implant surface materials
Authors: L Ji, T Wang, L Tian, H Song, M Gao
Mol Med Rep, 2019-11-12;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Unsaturated Aldehyde Acrolein Promotes Retinal Glial Cell Migration
Authors: M Murata, K Noda, S Yoshida, M Saito, A Fujiya, A Kanda, S Ishida
Invest. Ophthalmol. Vis. Sci., 2019-10-01;60(13):4425-4435.
Species: Human
Sample Types: Whole Tissue
Applications: IHC-P -
In Vivo Imaging of Small Molecular Weight Peptides for Targeted Renal Drug Delivery: A Study in Normal and Polycystic Kidney Diseased Mice
Authors: Stephen C. Lenhard, Allen McAlexander, Anthony Virtue, William Fieles, Tina Skedzielewski, Mary Rambo et al.
Journal of Pharmacology and Experimental Therapeutics
-
Hierarchical assembly and disassembly of a transcriptionally active RAG locus in CD4+CD8+ thymocytes
Authors: Abani Kanta Naik, Aaron T. Byrd, Aaron C.K. Lucander, Michael S. Krangel
Journal of Experimental Medicine
Species: Mouse
Sample Types: Chromatin
Applications: Chromatin Immunoprecipitation (Negative) -
Analysis of regulator of G-protein signalling 2 (RGS2) expression and function during prostate cancer progression
Authors: A Linder, M Hagberg Th, JE Damber, K Welén
Sci Rep, 2018-11-22;8(1):17259.
Species: Human
Sample Types: Whole Tissue
Applications: IHC-P -
A Lamina-Associated Domain Border Governs Nuclear Lamina Interactions, Transcription, and Recombination of the Tcrb Locus
Authors: S Chen, TR Luperchio, X Wong, EB Doan, AT Byrd, K Roy Choudh, KL Reddy, MS Krangel
Cell Rep, 2018-11-13;25(7):1729-1740.e6.
Species: Mouse
Sample Types: Cell Lysates
Applications: ChIP Control -
A Kaposi's Sarcoma-Associated Herpesvirus Infection Mechanism Is Independent of Integrins alpha 3 beta 1, alpha V beta 3, and alpha V beta 5
Authors: Allison Alwan TerBush, Florianne Hafkamp, Hee Jun Lee, Laurent Coscoy
Journal of Virology
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry Control -
Preservation of type H vessels and osteoblasts by enhanced preosteoclast platelet-derived growth factor type BB attenuates glucocorticoid-induced osteoporosis in growing mice
Authors: Ping Yang, Shan Lv, Yan Wang, Yi Peng, Zixing Ye, Zhuying Xia et al.
Bone
-
Ciliary parathyroid hormone signaling activates transforming growth factor-beta to maintain intervertebral disc homeostasis during aging
Authors: Liwei Zheng, Yong Cao, Shuangfei Ni, Huabin Qi, Zemin Ling, Xin Xu et al.
Bone Research
Species: Mouse
Sample Types: Whole Cells
Applications: Immunocytochemistry -
Cathelicidin-Derived Antimicrobial Peptides Inhibit Zika Virus Through Direct Inactivation and Interferon Pathway
Authors: Miao He, Hainan Zhang, Yuju Li, Guangshun Wang, Beisha Tang, Jeffrey Zhao et al.
Frontiers in Immunology
-
Genome-Wide Bimolecular Fluorescence Complementation-Based Proteomic Analysis ofToxoplasma gondiiROP18's Human Interactome Shows Its Key Role in Regulation of Cell Immunity and Apoptosis
Authors: J Xia, L Kong, LJ Zhou, SZ Wu, LJ Yao, C He, CY He, HJ Peng
Front Immunol, 2018-02-05;9(0):61.
Species: Primate - Chlorocebus aethiops (African Green Monkey)
Sample Types: Cell Lysates
Applications: Western Blot -
Oncogenic dependency on beta -catenin in liver cancer cell lines correlates with pathway activation
Authors: Zhihu Ding, Chaomei Shi, Lan Jiang, Tatiana Tolstykh, Hui Cao, Dinesh S. Bangari et al.
Oncotarget
Species: Human
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
MicroRNA-30b controls endothelial cell capillary morphogenesis through regulation of transforming growth factor beta 2
Authors: GA Howe, K Kazda, CL Addison
PLoS ONE, 2017-10-04;12(10):e0185619.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Cyclodextrin Reduces Cholesterol Crystal-Induced Inflammation by Modulating Complement Activation
Authors: SS Bakke, MH Aune, N Niyonzima, K Pilely, L Ryan, M Skjelland, P Garred, P Aukrust, B Halvorsen, E Latz, JK Damås, TE Mollnes, T Espevik
J. Immunol., 2017-08-30;0(0):.
Species: Human
Sample Types: Plasma
Applications: Flow Cytometry -
Interactions between fibroblastic reticular cells and B cells promote mesenteric lymph node lymphangiogenesis
Authors: L Kumar Dube, P Karempudi, SA Luther, B Ludewig, NL Harris
Nat Commun, 2017-08-28;8(1):367.
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
MiR-497?195 cluster regulates angiogenesis during coupling with osteogenesis by maintaining endothelial Notch and HIF-1? activity
Authors: M Yang, CJ Li, X Sun, Q Guo, Y Xiao, T Su, ML Tu, H Peng, Q Lu, Q Liu, HB He, TJ Jiang, MX Lei, M Wan, X Cao, XH Luo
Nat Commun, 2017-07-07;8(0):16003.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Phosphorylated STAT5 directly facilitates parvovirus B19 DNA replication in human erythroid progenitors through interaction with the MCM complex
Authors: SS Ganaie, W Zou, P Xu, X Deng, S Kleiboeker, J Qiu
PLoS Pathog., 2017-05-01;13(5):e1006370.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
The mitochondrial protein CHCHD2 primes the differentiation potential of human induced pluripotent stem cells to neuroectodermal lineages
Authors: L Zhu, A Gomez-Dura, G Saretzki, S Jin, K Tilgner, D Melguizo-S, G Anyfantis, J Al-Aama, L Vallier, P Chinnery, M Lako, L Armstrong
J. Cell Biol., 2016-10-17;215(2):187-202.
Species: Human
Sample Types: Protein
Applications: ChIP -
Dux4 controls migration of mesenchymal stem cells through the Cxcr4-Sdf1 axis
Authors: P Dmitriev, E Kiseleva, O Kharchenko, E Ivashkin, A Pichugin, P Dessen, T Robert, F Coppée, A Belayew, G Carnac, D Laoudj-Che, M Lipinski, A Vasiliev, YS Vassetzky
Oncotarget, 2016-10-04;7(40):65090-65108.
Species: Human
Sample Types: Whole Cells
Applications: Control -
Activity-Regulated Cytoskeleton-Associated Protein Controls AMPAR Endocytosis through a Direct Interaction with Clathrin-Adaptor Protein 2123
Authors: Luis L. P. DaSilva, Mark J. Wall, Luciana P. de Almeida, Sandrine C. Wauters, Yunan C. Januário, Jürgen Müller et al.
eNeuro
-
TGF-? Neutralization Enhances AngII-Induced Aortic Rupture and Aneurysm in Both Thoracic and Abdominal Regions
Authors: X Chen, DL Rateri, DA Howatt, A Balakrishn, JJ Moorleghen, LA Cassis, A Daugherty
PLoS ONE, 2016-04-22;11(4):e0153811.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Tumor-infiltrating monocytes/macrophages promote tumor invasion and migration by upregulating S100A8 and S100A9 expression in cancer cells
Authors: SY Lim, AE Yuzhalin, AN Gordon-Wee, RJ Muschel
Oncogene, 2016-04-18;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Interleukin-10 deficiency impairs regulatory T cell-derived neuropilin-1 functions and promotes Th1 and Th17 immunity
Authors: Shimin Wang, Xiang Gao, Guobo Shen, Wei Wang, Jingyu Li, Jingyi Zhao et al.
Scientific Reports
-
Neutrophils suppress intraluminal NK-mediated tumor cell clearance and enhance extravasation of disseminated carcinoma cells
Authors: A Spiegel, MW Brooks, S Houshyar, F Reinhardt, M Ardolino, E Fessler, MB Chen, JA Krall, J DeCock, IK Zervantona, A Iannello, Y Iwamoto, V Cortez-Ret, RD Kamm, MJ Pittet, DH Raulet, RA Weinberg
Cancer Discov, 2016-04-12;0(0):.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
Lipoprotein receptor-related protein 6 is required for parathyroid hormone-induced Sost suppression
Authors: Changjun Li, Weishan Wang, Liang Xie, Xianghang Luo, Xu Cao, Mei Wan
Annals of the New York Academy of Sciences
-
TGRL Lipolysis Products Induce Stress Protein ATF3 via the TGF-beta Receptor Pathway in Human Aortic Endothelial Cells
Authors: Larissa Eiselein, Tun Nyunt, Michael W. Lamé, Kit F. Ng, Dennis W. Wilson, John C. Rutledge et al.
PLOS ONE
-
EpCAM-Independent Enrichment of Circulating Tumor Cells in Metastatic Breast Cancer
Authors: Helen Schneck, Berthold Gierke, Frauke Uppenkamp, Bianca Behrens, Dieter Niederacher, Nikolas H. Stoecklein et al.
PLOS ONE
-
Convergence of cMyc and beta-catenin on Tcf7l1 enables endoderm specification.
Authors: Morrison G, Scognamiglio R, Trumpp A, Smith A
EMBO J, 2015-12-16;35(3):356-68.
Species: Mouse
Sample Types: Cell Lysates
Applications: ChIP -
miR-142-5p and miR-130a-3p are regulated by IL-4 and IL-13 and control profibrogenic macrophage program
Authors: Shicheng Su, Qiyi Zhao, Chonghua He, Di Huang, Jiang Liu, Fei Chen et al.
Nature Communications
Species: Human
Sample Types: Nuclear Extract
Applications: Bioassay Control -
Characterization of In Vitro Engineered Human Adipose Tissues: Relevant Adipokine Secretion and Impact of TNF-alpha.
Authors: Aubin K, Safoine M, Proulx M, Audet-Casgrain M, Côté J, Tetu F, Roy A, Fradette J
PLoS ONE, 2015-09-14;10(9):e0137612.
Species: Human
Sample Types: Whole Tissue
Applications: IHC Control -
Suppression of Hyperactive Immune Responses Protects against Nitrogen Mustard Injury.
Authors: Au L, Meisch J, Das L, Binko A, Boxer R, Wen A, Steinmetz N, Lu K
J Invest Dermatol, 2015-08-19;135(12):2971-81.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
A discrete chromatin loop in the mouse Tcra-Tcrd locus shapes the TCRdelta and TCRalpha repertoires.
Authors: Chen L, Carico Z, Shih H, Krangel M
Nat Immunol, 2015-08-10;16(10):1085-93.
Species: Mouse
Sample Types: Cell Lysates
Applications: ChIP -
Optimisations and Challenges Involved in the Creation of Various Bioluminescent and Fluorescent Influenza A Virus Strains for In Vitro and In Vivo Applications.
Authors: Spronken M, Short K, Herfst S, Bestebroer T, Vaes V, van der Hoeven B, Koster A, Kremers G, Scott D, Gultyaev A, Sorell E, de Graaf M, Barcena M, Rimmelzwaan G, Fouchier R
PLoS ONE, 2015-08-04;10(8):e0133888.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC Control -
Interleukin-22 is increased in multiple sclerosis patients and targets astrocytes.
Authors: Perriard G, Mathias A, Enz L, Canales M, Schluep M, Gentner M, Schaeren-Wiemers N, Du Pasquier R
J Neuroinflammation, 2015-06-16;12(0):119.
Species: Human
Sample Types: Whole Cells
Applications: IHC -
Stearoyl-CoA desaturase 1 and paracrine diffusible signals have a major role in the promotion of breast cancer cell migration induced by cancer-associated fibroblasts.
Authors: Angelucci C, Maulucci G, Colabianchi A, Iacopino F, D'Alessio A, Maiorana A, Palmieri V, Papi M, De Spirito M, Di Leone A, Masetti R, Sica G
Br J Cancer, 2015-04-16;112(10):1675-86.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization Control -
An anti-silencer- and SATB1-dependent chromatin hub regulates Rag1 and Rag2 gene expression during thymocyte development.
Authors: Hao B, Naik A, Watanabe A, Tanaka H, Chen L, Richards H, Kondo M, Taniuchi I, Kohwi Y, Kohwi-Shigematsu T, Krangel M
J Exp Med, 2015-04-06;212(5):809-24.
Species: Mouse
Sample Types: Whole Cells
Applications: Immunoprecipitation -
Discovery of molecular markers to discriminate corneal endothelial cells in the human body.
Authors: Yoshihara M, Ohmiya H, Hara S, Kawasaki S, Hayashizaki Y, Itoh M, Kawaji H, Tsujikawa M, Nishida K
PLoS ONE, 2015-03-25;10(3):e0117581.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
T-cell receptor alpha enhancer is inactivated in alphabeta T lymphocytes.
Authors: del Blanco B, Angulo U, Krangel M, Hernandez-Munain C
Proc Natl Acad Sci U S A, 2015-03-23;112(14):E1744-53.
Species: Mouse
Sample Types: Whole Cells
Applications: Immunoprecipitation -
HEB associates with PRC2 and SMAD2/3 to regulate developmental fates.
Authors: Yoon, Se-Jin, Foley, Joseph W, Baker, Julie C
Nat Commun, 2015-03-16;6(0):6546.
Species: Mouse
Sample Types: Cell Lysates
Applications: Co-Immunoprecipitation -
MicroRNA-188 regulates age-related switch between osteoblast and adipocyte differentiation.
Authors: Li, Chang-Ju, Cheng, Peng, Liang, Meng-Ke, Chen, Yu-Si, Lu, Qiong, Wang, Jin-Yu, Xia, Zhu-Ying, Zhou, Hou-De, Cao, Xu, Xie, Hui, Liao, Er-Yuan, Luo, Xiang-Ha
J Clin Invest, 2015-03-09;125(4):1509-22.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Bmi1 limits dilated cardiomyopathy and heart failure by inhibiting cardiac senescence.
Authors: Gonzalez-Valdes I, Hidalgo I, Bujarrabal A, Lara-Pezzi E, Padron-Barthe L, Garcia-Pavia P, Gomez-del Arco P, Redondo J, Ruiz-Cabello J, Jimenez-Borreguero L, Enriquez J, de la Pompa J, Hidalgo A, Gonzalez S
Nat Commun, 2015-03-09;6(0):6473.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Nerve growth factor and proNGF simultaneously promote symmetric self-renewal, quiescence, and epithelial to mesenchymal transition to enlarge the breast cancer stem cell compartment.
Authors: Tomellini E, Touil Y, Lagadec C, Julien S, Ostyn P, Ziental-Gelus N, Meignan S, Lengrand J, Adriaenssens E, Polakowska R, Le Bourhis X
Stem Cells, 2015-02-01;33(2):342-53.
Species: Human
Sample Types: Whole Cells
Applications: ICC Control -
A transient wave of BMP signaling in the retina is necessary for Muller glial differentiation.
Authors: Ueki Y, Wilken M, Cox K, Chipman L, Bermingham-McDonogh O, Reh T
Development, 2015-02-01;142(3):533-43.
Species: Mouse
Sample Types: Whole Cells
Applications: ChIP -
Regulation of caspase-3 processing by cIAP2 controls the switch between pro-inflammatory activation and cell death in microglia
Authors: E Kavanagh, J Rodhe, M A Burguillos, J L Venero, B Joseph
Cell Death & Disease
-
Specification of Vdelta and Valpha usage by Tcra/Tcrd locus V gene segment promoters.
Authors: Naik A, Hawwari A, Krangel M
J Immunol, 2014-12-03;194(2):790-4.
Species: Mouse
Sample Types: Cell Lysates
Applications: ChIP Control -
The heparan sulfate proteoglycan agrin contributes to barrier properties of mouse brain endothelial cells by stabilizing adherens junctions
Authors: Esther Steiner, Gaby U. Enzmann, Ruth Lyck, Shuo Lin, Markus A. Rüegg, Stephan Kröger et al.
Cell and Tissue Research
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
The CLEC-2-podoplanin axis controls the contractility of fibroblastic reticular cells and lymph node microarchitecture.
Authors: Astarita, Jillian, Cremasco, Viviana, Fu, Jianxin, Darnell, Max C, Peck, James R, Nieves-Bonilla, Janice M, Song, Kai, Kondo, Yuji, Woodruff, Matthew, Gogineni, Alvin, Onder, Lucas, Ludewig, Burkhard, Weimer, Robby M, Carroll, Michael, Mooney, David J, Xia, Lijun, Turley, Shannon
Nat Immunol, 2014-10-27;16(1):75-84.
Species: Mouse
Sample Types: Whole Cells
Applications: Control -
CD4(+)Foxp3(+) Tregs protect against innate immune cell-mediated fulminant hepatitis in mice.
Authors: Hou X, Song J, Su J, Huang D, Gao W, Yan J, Shen J
Mol Immunol, 2014-10-12;63(2):420-7.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization Control -
S100A4 is upregulated in proliferative diabetic retinopathy and correlates with markers of angiogenesis and fibrogenesis
Authors: Ahmed M. Abu El-Asrar, Mohd Imtiaz Nawaz, Gert De Hertogh, Kaiser Alam, Mohammad Mairaj Siddiquei, Kathleen Van den Eynde et al.
Mol. Vis
-
Tumour-derived PTH-related protein triggers adipose tissue browning and cancer cachexia
Authors: Serkan Kir, James P. White, Sandra Kleiner, Lawrence Kazak, Paul Cohen, Vickie E. Baracos et al.
Nature
-
Th17-type cytokines, IL-6 and TNF-alpha synergistically activate STAT3 and NF-kB to promote colorectal cancer cell growth.
Authors: De Simone V, Franze E, Ronchetti G, Colantoni A, Fantini M, Di Fusco D, Sica G, Sileri P, MacDonald T, Pallone F, Monteleone G, Stolfi C
Oncogene, 2014-09-01;34(27):3493-503.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Proteomic analysis of the action of the Mycobacterium ulcerans toxin mycolactone: targeting host cells cytoskeleton and collagen.
Authors: Gama J, Ohlmeier S, Martins T, Fraga A, Sampaio-Marques B, Carvalho M, Proenca F, Silva M, Pedrosa J, Ludovico P
PLoS Negl Trop Dis, 2014-08-07;8(8):e3066.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Differential Requirement for P2X7R Function in IL-17 Dependent vs. IL-17 Independent Cellular Immune Responses
Authors: J.A. Sullivan, E. Jankowska-Gan, L. Shi, D. Roenneburg, S. Hegde, D.S. Greenspan et al.
American Journal of Transplantation
-
Fibrodysplasia ossificans progressiva-related activated activin-like kinase signaling enhances osteoclast formation during heterotopic ossification in muscle tissues.
Authors: Yano M, Kawao N, Okumoto K, Tamura Y, Okada K, Kaji H
J Biol Chem, 2014-05-05;289(24):16966-77.
Species: Mouse
Sample Types: Whole Cells
Applications: Isotype Control -
In vivo neuronal action potential recordings via three-dimensional microscale needle-electrode arrays.
Authors: Fujishiro A, Kaneko H, Kawashima T, Ishida M, Kawano T
Sci Rep, 2014-05-02;4(0):4868.
Species: Rat
Sample Types: Whole Tissue
Applications: IHC-Fr -
NADPH oxidase-independent formation of extracellular DNA traps by basophils.
Authors: Morshed M, Hlushchuk R, Simon D, Walls A, Obata-Ninomiya K, Karasuyama H, Djonov V, Eggel A, Kaufmann T, Simon H, Yousefi S
J Immunol, 2014-04-25;192(11):5314-23.
Species: Mouse
Sample Types: Whole Cells
Applications: Isotype Control -
Cholesterol crystals induce complement-dependent inflammasome activation and cytokine release.
Authors: Samstad E, Niyonzima N, Nymo S, Aune M, Ryan L, Bakke S, Lappegard K, Brekke O, Lambris J, Damas J, Latz E, Mollnes T, Espevik T
J Immunol, 2014-02-19;192(6):2837-45.
Species: Human
Sample Types:
Applications: Isotype Control -
IL-33 targeting attenuates intestinal mucositis and enhances effective tumor chemotherapy in mice.
Authors: Guabiraba R, Besnard A, Menezes G, Secher T, Jabir M, Amaral S, Braun H, Lima-Junior R, Ribeiro R, Cunha F, Teixeira M, Beyaert R, Graham G, Liew F
Mucosal Immunol, 2014-01-15;7(5):1079-93.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Autophagy fosters myofibroblast differentiation through MTORC2 activation and downstream upregulation of CTGF.
Authors: Bernard M, Dieude M, Yang B, Hamelin K, Underwood K, Hebert M
Autophagy, 2014-01-01;10(12):2193-207.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
A soluble fucose-specific lectin from Aspergillus fumigatus conidia--structure, specificity and possible role in fungal pathogenicity.
Authors: Houser J, Komarek J, Kostlanova N, Cioci G, Varrot A, Kerr S, Lahmann M, Balloy V, Fahy J, Chignard M, Imberty A, Wimmerova M
PLoS ONE, 2013-12-10;8(12):e83077.
Species: Fungus - Aspergillus fumigatus
Sample Types: Whole Tissue
Applications: IHC -
FGF10 Signaling differences between type I pleuropulmonary blastoma and congenital cystic adenomatoid malformation
Authors: Guillaume Lezmi, Virginie Verkarre, Naziha Khen-Dunlop, Shamila Vibhushan, Alice Hadchouel, Caroline Rambaud et al.
Orphanet Journal of Rare Diseases
Species: Human
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Characterization of dextran sodium sulfate-induced inflammation and colonic tumorigenesis in Smad3(-/-) mice with dysregulated TGF beta
Authors: Audrey Seamons, Piper M. Treuting, Thea Brabb, Lillian Maggio-Price
PLoS ONE
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
WISP1/CCN4: A Potential Target for Inhibiting Prostate Cancer Growth and Spread to Bone
Authors: Mitsuaki Ono, Colette A. Inkson, Robert Sonn, Tina M. Kilts, Luis F. de Castro, Azusa Maeda et al.
PLoS ONE
-
Developmentally programmed 3' CpG island methylation confers tissue- and cell-type-specific transcriptional activation.
Authors: Yu, Da-Hai, Ware, Carol, Waterland, Robert A, Zhang, Jiexin, Chen, Miao-Hsu, Gadkari, Manasi, Kunde-Ramamoorthy, Govindar, Nosavanh, Lagina M, Shen, Lanlan
Mol Cell Biol, 2013-03-04;33(9):1845-58.
Species: Human
Sample Types: Cell Lysates
Applications: Immunoprecipitation -
Tcra gene recombination is supported by a Tcra enhancer- and CTCF-dependent chromatin hub
Authors: Han-Yu Shih, Jiyoti Verma-Gaur, Ali Torkamani, Ann J. Feeney, Niels Galjart, Michael S. Krangel
Proceedings of the National Academy of Sciences
-
The miR-17/92 cluster is targeted by STAT5 but dispensable for mammary development
Authors: Yonatan Feuermann, Gertraud W. Robinson, Bing-Mei Zhu, Keunsoo Kang, Noa Raviv, Daisuke Yamaji et al.
genesis
-
CX3CL1 expression in the conjunctiva is involved in immune cell trafficking during toxic ocular surface inflammation.
Authors: Denoyer A, Godefroy D, Celerier I, Frugier J, Riancho L, Baudouin F, Rostene W, Baudouin C
Mucosal Immunol, 2012-06-13;5(0):702.
Species: Human
Sample Types: Whole Cells
Applications: Control -
Genome-wide analyses reveal the extent of opportunistic STAT5 binding that does not yield transcriptional activation of neighboring genes
Authors: Bing-Mei Zhu, Keunsoo Kang, Ji Hoon Yu, Weiping Chen, Harold E. Smith, Daeyoup Lee et al.
Nucleic Acids Research
-
Regulation of T cell receptor beta allelic exclusion by gene segment proximity and accessibility1,2
Authors: Hrisavgi D. Kondilis-Mangum, Han-Yu Shih, Grace Mahowald, Barry P. Sleckman, Michael S. Krangel
The Journal of Immunology
-
Transforming Growth Factor Beta Expression by Human Vascular Cells Inhibits Interferon Gamma Production and Arterial Media Injury by Alloreactive Memory T Cells
Authors: Amir H. Lebastchi, Salman F. Khan, Lingfeng Qin, Wei Li, Jing Zhou, Narutoshi Hibino et al.
American Journal of Transplantation
-
Long-distance regulation of fetal Vδ gene segment TRDV4 by the Tcrd enhancer1,2
Authors: Bingtao Hao, Michael S. Krangel
The Journal of Immunology
-
A barrier-type insulator forms a boundary between active and inactive chromatin at the murine T cell receptor beta locus1,2
Authors: Juan Carabana, Akiko Watanabe, Bingtao Hao, Michael S. Krangel
The Journal of Immunology
Species: Transgenic Mouse
Sample Types: Chromatin
Applications: Chromatin Immunoprecipitation (ChIP) -
MEK5 is Activated by Shear Stress, Activates ERK5 and Induces KLF4 to Modulate TNF Responses in Human Dermal Microvascular Endothelial Cells
Authors: PAUL R. CLARK, TODD J. JENSEN, MARTIN S. KLUGER, MAURICE MORELOCK, ADEDAYO HANIDU, ZHENHAO QI et al.
Microcirculation
-
Proteolytic release of the receptor for advanced glycation end products from in vitro and in situ alveolar epithelial cells.
Authors: Yamakawa N, Uchida T, Matthay MA, Makita K
Am. J. Physiol. Lung Cell Mol. Physiol., 2011-01-21;300(4):L516-25.
Species: Rat
Sample Types: Whole Cells
Applications: Control -
A requirement for Lim domain binding protein 1 in erythropoiesis
Authors: LiQi Li, Jan Y. Lee, Jennifer Gross, Sang-Hyun Song, Ann Dean, Paul E. Love
Journal of Experimental Medicine
-
Stemness markers characterize IGR-CaP1, a new cell line derived from primary epithelial prostate cancer.
Authors: Chauchereau A, Al Nakouzi N, Gaudin C, Le Moulec S, Compagno D, Auger N, Benard J, Opolon P, Rozet F, Validire P, Fromont G, Fizazi K
Exp. Cell Res., 2010-10-23;317(3):262-75.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry Control -
The sensing of environmental stimuli by follicular dendritic cells promotes immunoglobulin A generation in the gut.
Authors: Suzuki K, Maruya M, Kawamoto S
Immunity, 2010-07-23;33(1):71-83.
Species: Mouse
Sample Types: Whole Cells
Applications: Control -
Stromal regulation of vessel stability by MMP14 and TGFbeta
Authors: Nor E. Sounni, Kerstin Dehne, Leon van Kempen, Mikala Egeblad, Nesrine I. Affara, Ileana Cuevas et al.
Disease Models & Mechanisms
Species: Mouse, Transgenic Mouse
Sample Types: In Vivo
Applications: Neutralization Control -
Tumor necrosis factor alpha as a new target for renal cell carcinoma: two sequential phase II trials of infliximab at standard and high dose.
Authors: Harrison ML, Obermueller E, Maisey NR, Hoare S, Edmonds K, Li NF, Chao D, Hall K, Lee C, Timotheadou E, Charles K, Ahern R, King DM, Eisen T, Corringham R, DeWitte M, Balkwill F, Gore M
J. Clin. Oncol., 2007-10-10;25(29):4542-9.
Species: Human
Sample Types: Whole Tissue
Applications: Control -
Endothelial damage from cytomegalovirus-specific host immune response can be prevented by targeted disruption of fractalkine-CX3CR1 interaction.
Authors: Bolovan-Fritts CA, Spector SA
Blood, 2007-09-25;111(1):175-82.
Species: Human
Sample Types: Whole Cells
Applications: Control -
Increase in transforming growth factor-beta in the brain during infection is related to fever, not depression of spontaneous motor activity.
Authors: Matsumura S, Shibakusa T, Fujikawa T, Yamada H, Inoue K, Fushiki T
Neuroscience, 2006-12-06;144(3):1133-40.
Species: Rat
Sample Types: CSF
Applications: Control -
Metastable tolerance to rhesus monkey renal transplants is correlated with allograft TGF-beta 1+CD4+ T regulatory cell infiltrates.
Authors: Torrealba JR, Katayama M, Fechner JH, Jankowska-Gan E, Kusaka S, Schultz JM, Hu H, Hamawy MM, Jonker M, Wubben J, Doxiadis G, Bontrop R, Burlingham WJ, Knechtle SJ
J. Immunol., 2004-05-01;172(9):5753-64.
Species: Primate - Macaca mulatta (Rhesus Macaque)
Sample Types: Whole Cells
Applications: Control -
Natural and induced CD4+CD25+ cells educate CD4+CD25- cells to develop suppressive activity: the role of IL-2, TGF-beta, and IL-10.
Authors: Zheng SG, Wang JH, Gray JD, Soucier H, Horwitz DA
J. Immunol., 2004-05-01;172(9):5213-21.
Species: Human
Sample Types: Whole Cells
Applications: Control -
Wnt regulation of progenitor maturation in the cortex depends on Shh or fibroblast growth factor 2.
Authors: Viti J, Gulacsi A, Lillien L
J. Neurosci., 2003-07-02;23(13):5919-27.
Species: Mouse
Sample Types: Whole Tissue
Applications: Control -
Fibrinolysis protease receptors promote activation of astrocytes to express pro-inflammatory cytokines
Authors: P Pontecorvi, MA Banki, C Zampieri, C Zalfa, P Azmoon, MZ Kounnas, C Marchese, SL Gonias, E Mantuano
J Neuroinflammation, 2019-12-06;16(1):257.
-
Sialidase inhibitors attenuate pulmonary fibrosis in a mouse model
Authors: TR Karhadkar, D Pilling, N Cox, RH Gomer
Sci Rep, 2017-11-08;7(1):15069.
-
Surfaceome Profiling of Cell Lines and Patient-Derived Xenografts Confirm FGFR4, NCAM1, CD276, and Highlight AGRL2, JAM3, and L1CAM as Surface Targets for Rhabdomyosarcoma
Authors: A Timpanaro, C Piccand, AC Uldry, PK Bode, D Dzhumashev, R Sala, M Heller, J Rössler, M Bernasconi
International Journal of Molecular Sciences, 2023-01-30;24(3):.
-
Proximity Ligation Assay Detection of Protein-DNA Interactions-Is There a Link between Heme Oxygenase-1 and G-quadruplexes?
Authors: Krzeptowski W, Chudy P, Sokołowski G, et al.
Antioxidants (Basel, Switzerland)
-
Myc CoopeRates with Ras by Programming Inflammation and Immune Suppression.
Authors: Kortlever Roderik M, Sodir Nicole M, Wilson Catherine H et al.
Cell
-
The long non-coding RNA nuclear-enriched abundant transcript 1_2 induces paraspeckle formation in the motor neuron during the early phase of amyotrophic lateral sclerosis.
Authors: Nishimoto Y, Nakagawa S, Hirose T et al.
Mol Brain.
FAQs
No product specific FAQs exist for this product, however you may
View all Isotype Control FAQsReviews for Normal Rabbit IgG Control
Average Rating: 4.4 (Based on 11 Reviews)
Have you used Normal Rabbit IgG Control?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:




