Mouse/Rat Tie-2 Antibody Summary
Ala23-Lys744
Accession # CAA47857
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
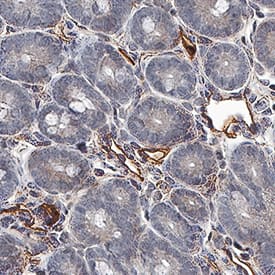
Tie-2 in Mouse Intestine Tissue. Tie-2 was detected in immersion fixed paraffin-embedded sections of mouse intestine tissue using Goat Anti-Mouse/Rat Tie-2 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF762) at 1 µg/mL for 1 hour at room temperature followed by incubation with the Anti-Goat IgG VisUCyte™ HRP Polymer Antibody (Catalog # VC004). Before incubation with the primary antibody, tissue was subjected to heat-induced epitope retrieval using Antigen Retrieval Reagent-Basic (Catalog # CTS013). Tissue was stained using DAB (brown) and counterstained with hematoxylin (blue). Specific staining was localized to cell surface on endothelial cells. View our protocol for IHC Staining with VisUCyte HRP Polymer Detection Reagents.
 View Larger
View Larger
Detection of Mouse Tie-2 by Western Blot Pericytes express functional Tie2.(a) Microarray-based expression screening of brain pericytes (BP), placenta pericytes (PP), pancreas pericytes (PA), lung pericytes (LP), muscle pericytes (MP) and human umbilical vein endothelial cells (HUVEC [HU]). (b) Semi-quantitative PCR of TEK (Tie2), endothelial marker genes (PECAM1, CDH5) and pericyte marker genes (CSPG4, CD248) in HUVEC and BP; house keeping gene: GAPDH. (c) Representative images showing expression of Tie2 in HUVEC and BP. (d) Histogram of membrane-bound Tie2 expression in HUVEC and BP compared to isotype control measured by flow cytometry. (e,g) Western blot (WB) analysis of tyrosine phosphorylation (pTyr) and total Tie2 after Tie2 immunoprecipitation (IP) in BP (e) and HUVEC (g) upon stimulation with recombinant human (rh) Ang1 compared to unstimulated (us) cells. (f,h) Quantification of the ratio of phosphorylated Tie2 (pTie2) relative to total Tie2 protein in BP (f) and HUVEC (h) normalized to us control (n=3). (i,k) Western blot analysis of AKT phosphorylation (Ser473) in control (shCtr) and Tie2-silenced (shTie2 I/shTie2 II) BP (i) and HUVEC (k) upon stimulation with rhAng1 or rhAng2. (j,l) Quantification of the ratio of pAKT to total AKT protein expression in BP (j) and HUVEC (l) normalized to unstimulated control (n=3). Representative western blot images are cropped versions and original images can be found in Supplementary Fig. 15. Scale bars: 20 μm (c). Data are shown as mean±s.d. Statistics were performed using Mann–Whitney U test (f,h) and one-way ANOVA (j,l). *P<0.05, **P<0.01. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/28719590), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Tie-2 by Immunocytochemistry/ Immunofluorescence Endothelial changes after pericyte depletion. a–f Maximum intensity projection of confocal images from control and DTRiPC P6 retinas stained for IB4 (red) in combination with VEGF-A a, VEGFR2 b, VEGFR3 c, Tie2 d, Esm1 e, and Dll4 f (all in white), as indicated. Note local increase of VEGFR2, VEGFR3, and Esm1 (arrowheads in b, c, e) but not Tie2 or VEGF-A at the edge of the vessel plexus. Dll4 expression in DTRiPC sprouts is increased in some regions (arrowheads) but absent in others (arrows). Scale bar, 100 µm. g–j Quantitation of VEGF-A immunosignals area and intensity g, signal intensity for VEGFR2 h and VEGFR3 i and proportion of Esm1+ area with respect to vascular area j in the P6 control and DTRiPC angiogenic front. Error bars, s.e.m. p-values, Student’s t-test. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/29146905), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Tie-2 by Western Blot Pericyte-derived Angpt1 controls alveologenesis. a RT-qPCR analysis of Angpt1 and Tie2/Tek expression in freshly sorted lung GFP+, CD31+ or EpCAM+ cells from P7 Pdgfrb(BAC)-CreERT2 R26-mT/mG mice. Data represents mean ± s.e.m. (n = 4 mice). b High magnification images of P10 Angpt1GFP lungs stained for GFP (green), PDGFR beta (red), and PDGFR alpha (blue). Arrows indicate GFP and PDGFR beta double positive pericytes. Scale bar, 15 µm. c RT-qPCR analysis of Angpt1 expression in freshly sorted PDGFR beta + cells from P7 Yap1,Wwtr1iPCKO and control lungs. Data represents mean ± s.e.m. (n = 4 mice, two-tailed unpaired t-test). dAngpt1 expression in cultured Verteporfin (VP)-treated (48 h) and control pericytes. Data represents mean ± s.e.m. (n = 4, Welch’s t-test). e Expression of the indicated transcripts in freshly sorted CD31+ cells from P7 Yap1,Wwtr1iPCKO and control lungs. Data represents mean ± s.e.m. (n = 4 mice, NS not significant, two-tailed unpaired t-test). f–h Western blot analysis of Angpt1 protein (f; n = 2 controls and 4 mutant mice) and of total and phospho-Tie2 (pTie2) in P12 Yap1,Wwtr1iPCKO and control total lung lysates (g, n = 3 controls and 5 mutants). Molecular weight marker (kDa) is indicated. Relative quantification of signals is shown in h. Two-tailed unpaired t-test. i Scheme showing the time points of tamoxifen administration and analysis for Angpt1iPCKO mice. j, k 3D reconstruction confocal images of P12 Angpt1iPCKO and littermate control lungs stained for AQP5 (green), PDGFR beta (red), and PECAM1 (blue). Panels in k show higher magnification of PECAM1 staining. Scale bar, 50 µm (j) and 30 µm (k). l Quantitation of airspace volume in P12 Angpt1iPCKO and littermate control lung sections with 3D reconstruction surface images. Data represents mean ± s.e.m. (n = 4 mice; p < 0.0001, two-tailed unpaired t-test). m 3D reconstruction confocal images of P12 Angpt1iPCKO and littermate control lungs stained for alpha SMA (red) and tropoelastin (blue). Scale bar, 50 µm. n Quantitation of staining intensity for alpha SMA or tropoelastin shown in m. Intensity was normalized to the size of the parenchymal region. Data represents mean ± s.e.m. (n = 4 mice; NS not significant, two-tailed unpaired t-test). o Schematic summary of findings. Pulmonary pericytes regulate ECs and alveolar epithelial cells via angiocrine factors such as angiopoietin-1 and HGF. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/29934496), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Tie-2 by Western Blot Pericytes express functional Tie2.(a) Microarray-based expression screening of brain pericytes (BP), placenta pericytes (PP), pancreas pericytes (PA), lung pericytes (LP), muscle pericytes (MP) and human umbilical vein endothelial cells (HUVEC [HU]). (b) Semi-quantitative PCR of TEK (Tie2), endothelial marker genes (PECAM1, CDH5) and pericyte marker genes (CSPG4, CD248) in HUVEC and BP; house keeping gene: GAPDH. (c) Representative images showing expression of Tie2 in HUVEC and BP. (d) Histogram of membrane-bound Tie2 expression in HUVEC and BP compared to isotype control measured by flow cytometry. (e,g) Western blot (WB) analysis of tyrosine phosphorylation (pTyr) and total Tie2 after Tie2 immunoprecipitation (IP) in BP (e) and HUVEC (g) upon stimulation with recombinant human (rh) Ang1 compared to unstimulated (us) cells. (f,h) Quantification of the ratio of phosphorylated Tie2 (pTie2) relative to total Tie2 protein in BP (f) and HUVEC (h) normalized to us control (n=3). (i,k) Western blot analysis of AKT phosphorylation (Ser473) in control (shCtr) and Tie2-silenced (shTie2 I/shTie2 II) BP (i) and HUVEC (k) upon stimulation with rhAng1 or rhAng2. (j,l) Quantification of the ratio of pAKT to total AKT protein expression in BP (j) and HUVEC (l) normalized to unstimulated control (n=3). Representative western blot images are cropped versions and original images can be found in Supplementary Fig. 15. Scale bars: 20 μm (c). Data are shown as mean±s.d. Statistics were performed using Mann–Whitney U test (f,h) and one-way ANOVA (j,l). *P<0.05, **P<0.01. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/28719590), licensed under a CC-BY license. Not internally tested by R&D Systems.
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: Tie-2
Tie-1/Tie (tyrosine kinase with Ig and EGF homology domains 1) and Tie-2/Tek comprise a receptor tyrosine kinase (RTK) subfamily with unique structural characteristics: two immunoglobulin-like domains flanking three epidermal growth factor (EGF)-like domains, followed by three fibronectin type III-like repeats in the extracellular region and a split tyrosine kinase domain in the cytoplasmic region. These receptors are expressed primarily on endothelial and hematopoietic progenitor cells and play critical roles in angiogenesis, vasculogenesis and hematopoiesis.
Two ligands, angiopoietin-1 (Ang-1) and angiopoietin-2 (Ang-2), which bind Tie-2 with high-affinity have been identified. Ang-2 has been reported to act as an antagonist for Ang-1. Mice engineered to overexpress Ang-2 or to lack Ang-1 or Tie-2 display similar angiogenesis defects.
- Partanen, J. and D.J. Dumont (1999) Curr. Top. Microbiol. Immunol. 237:159.
- Takakura, N. et al. (1998) Immunity 9:677.
Product Datasheets
Citations for Mouse/Rat Tie-2 Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
38
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
miR-15a/-16 Inhibit Angiogenesis by Targeting the Tie2 Coding Sequence: Therapeutic Potential of a miR-15a/16 Decoy System in Limb Ischemia
Authors: Marie Besnier, Saran Shantikumar, Maryam Anwar, Parul Dixit, Aranzazu Chamorro-Jorganes, Walid Sweaad et al.
Molecular Therapy - Nucleic Acids
-
Integrin ?3/Akt signaling contributes to platelet-induced hemangioendothelioma growth
Authors: R Gu, X Sun, Y Chi, Q Zhou, H Xiang, DB Bosco, X Lai, C Qin, KF So, Y Ren, XM Chen
Sci Rep, 2017-07-25;7(1):6455.
-
The effect of targeting Tie2 on hemorrhagic shock-induced renal perfusion disturbances in rats
Authors: Anoek L. I. van Leeuwen, Nicole A. M. Dekker, Paul Van Slyke, Esther de Groot, Marc G. Vervloet, Joris J. T. H. Roelofs et al.
Intensive Care Medicine Experimental
-
Preclinical validation of a novel metastasis-inhibiting Tie1 function-blocking antibody
Authors: M Singhal, N Gengenbach, S La Porta, S Gehrs, J Shi, M Kamiyama, DM Bodenmille, A Fischl, B Schieb, E Besemfelde, S Chintharla, HG Augustin
EMBO Mol Med, 2020-04-17;0(0):e11164.
-
Patho-mechanisms for hemorrhage/sepsis-induced indirect acute respiratory distress syndrome (iARDS): A role for lung Tie1 and its regulation by neutrophils
Authors: Jiali Zhu, Jinbao Li, Chun-Shiang Chung, Joanne L. Lomas-Neira, Alfred Ayala
Shock
-
Angiopoietin-2 blockade ameliorates autoimmune neuroinflammation by inhibiting leukocyte recruitment into the CNS
Authors: Z Li, EA Korhonen, A Merlini, J Strauss, E Wihuri, H Nurmi, S Antila, J Paech, U Deutsch, B Engelhardt, S Chintharla, GY Koh, A Flügel, K Alitalo
J. Clin. Invest., 2020-04-01;0(0):.
-
Endothelial deletion of EPH receptor A4 alters single-cell profile and Tie2/Akap12 signaling to preserve blood-brain barrier integrity
Authors: Cash, A;de Jager, C;Brickler, T;Soliman, E;Ladner, L;Kaloss, AM;Zhu, Y;Pridham, KJ;Mills, J;Ju, J;Basso, EKG;Chen, M;Johnson, Z;Sotiropoulos, Y;Wang, X;Xie, H;Matson, JB;Marvin, EA;Theus, MH;
Proceedings of the National Academy of Sciences of the United States of America
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Shape-memory collagen scaffold combined with hyaluronic acid for repairing intervertebral disc
Authors: YW Koo, CS Lim, A Darai, J Lee, W Kim, I Han, GH Kim
Biomaterials research, 2023-03-29;27(1):26.
Species: Rat
Sample Types: Whole Tissue
Applications: IHC -
An agonistic anti-Tie2 antibody suppresses the normal-to-tumor vascular transition in the glioblastoma invasion zone
Authors: E Lee, EA Lee, E Kong, H Chon, M Llaiqui-Co, CH Park, BY Park, NR Kang, JS Yoo, HS Lee, HS Kim, SH Park, SW Choi, D Vestweber, JH Lee, P Kim, WS Lee, I Kim
Experimental & Molecular Medicine, 2023-02-24;55(2):470-484.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Shear stress control of vascular leaks and atheromas through Tie2 activation by VE-PTP sequestration
Authors: K Shirakura, P Baluk, AF Nottebaum, U Ipe, KG Peters, DM McDonald, D Vestweber
Embo Molecular Medicine, 2023-02-06;0(0):e16128.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Adjunctive therapy with the Tie2 agonist Vasculotide reduces pulmonary permeability in Streptococcus pneumoniae infected and mechanically ventilated mice
Authors: A Lask, B Gutbier, O Kershaw, G Nouailles, AD Gruber, HC Müller-Red, S Chackowicz, DA Hamilton, P Van Slyke, M Witzenrath
Scientific Reports, 2022-09-15;12(1):15531.
Species: Mouse
Sample Types: Tissue Homogenates
Applications: Western Blot -
Monocyte pro-inflammatory phenotypic control by Ephrin type-A receptor 4 mediates neural tissue damage
Authors: EA Kowalski, E Soliman, C Kelly, EK Gudenschwa, J Leonard, KJ Pridham, J Ju, AM Cash, A Hazy, C de Jager, AM Kaloss, H Ding, RD Hernandez, GM Coleman, X Wang, ML Olsen, AM Pickrell, MH Theus
JCI Insight, 2022-08-08;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
EphB4 and ephrinB2 act in opposition in the head and neck tumor microenvironment
Authors: S Bhatia, D Nguyen, LB Darragh, B Van Court, J Sharma, MW Knitz, M Piper, S Bukkapatna, J Gadwa, TE Bickett, S Bhuvane, S Corbo, B Wu, Y Lee, M Fujita, M Joshi, LE Heasley, RL Ferris, O Rodriguez, C Albanese, M Kapoor, EB Pasquale, SD Karam
Nature Communications, 2022-06-20;13(1):3535.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Pericyte Loss Leads to Capillary Stalling Through Increased Leukocyte-Endothelial Cell Interaction in the Brain
Authors: YG Choe, JH Yoon, J Joo, B Kim, SP Hong, GY Koh, DS Lee, WY Oh, Y Jeong
Frontiers in Cellular Neuroscience, 2022-03-11;16(0):848764.
Species: Mouse
Sample Types: Protein Lysates, Whole Tissue
Applications: IHC, Western Blot -
An autophagic deficit in the uterine vessel microenvironment provokes hyperpermeability through deregulated VEGFA, NOS1, and CTNNB1
Authors: B Lee, H Shin, JE Oh, J Park, M Park, SC Yang, JH Jun, SH Hong, H Song, HJ Lim
Autophagy, 2020-06-17;0(0):1-18.
Species: Mouse
Sample Types: Tissue Homogenates
Applications: Western Blot -
Role of the Ang2-Tie2 Axis in Vascular Damage Driven by High Glucose or Nucleoside Diphosphate Kinase B Deficiency
Authors: A Chatterjee, R Eshwaran, H Huang, D Zhao, M Schmidt, T Wieland, Y Feng
Int J Mol Sci, 2020-05-25;21(10):.
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: IHC -
Perivascular Stem Cell-Derived Cyclophilin A Improves Uterine Environment with Asherman's Syndrome via HIF1alpha-Dependent Angiogenesis
Authors: M Park, SH Hong, SH Park, YS Kim, SC Yang, HR Kim, S Noh, S Na, HK Lee, HJ Lim, SW Lyu, H Song
Mol. Ther., 2020-05-20;0(0):.
Species: Mouse
Sample Types: Tissue Homogenates
Applications: Western Blot -
Mesenchymal-endothelial transition-derived cells as a potential new regulatory target for cardiac hypertrophy
Authors: W Dong, R Li, H Yang, Y Lu, L Zhou, L Sun, D Wang, J Duan
Sci Rep, 2020-04-20;10(1):6652.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
C16 Peptide Promotes Vascular Growth and Reduces Inflammation in a Neuromyelitis Optica Model
Authors: H Chen, X Fu, J Jiang, S Han
Front Pharmacol, 2019-12-03;10(0):1373.
Species: Rat
Sample Types: Tissue Homogenates
Applications: Western Blot -
miR-15a/-16 Inhibit Angiogenesis by Targeting the Tie2 Coding Sequence: Therapeutic Potential of a miR-15a/16 Decoy System in Limb Ischemia
Authors: Marie Besnier, Saran Shantikumar, Maryam Anwar, Parul Dixit, Aranzazu Chamorro-Jorganes, Walid Sweaad et al.
Molecular Therapy - Nucleic Acids
Species: Mouse
Sample Types: Tissue Homogenates
Applications: Western Blot -
Tumor angiogenesis is differentially regulated by phosphorylation of endothelial cell focal adhesion kinase tyrosines-397 and -861
Authors: AR Pedrosa, N Bodrug, J Gomez-Escu, EP Carter, LE Reynolds, PN Georgiou, I Fernandez, DM Lees, V Kostourou, AN Alexopoulo, S Batista, B Tavora, B Serrels, M Parsons, T Iskratsch, KM Hodivala-D
Cancer Res., 2019-06-12;0(0):.
Species: Mouse, Transgenic Mouse
Sample Types: Cell Lysates, Whole Tissue
Applications: IHC, Western Blot -
Tie2 activation promotes choriocapillary regeneration for alleviating neovascular age-related macular degeneration
Authors: J Kim, JR Park, J Choi, I Park, Y Hwang, H Bae, Y Kim, W Choi, JM Yang, S Han, TY Chung, P Kim, Y Kubota, HG Augustin, WY Oh, GY Koh
Sci Adv, 2019-02-13;5(2):eaau6732.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Pulmonary pericytes regulate lung morphogenesis
Authors: K Kato, R Diéguez-Hu, DY Park, SP Hong, S Kato-Azuma, S Adams, M Stehling, B Trappmann, JL Wrana, GY Koh, RH Adams
Nat Commun, 2018-06-22;9(1):2448.
Species: Mouse
Sample Types: Cell Lysates
Applications: Western Blot -
Tie2 protects the vasculature against thrombus formation in systemic inflammation
Authors: SJ Higgins, K Ceunynck, J Kellum, X Chen, X Gu, SA Chaudhry, S Schulman, TA Libermann, S Lu, NI Shapiro, DC Christiani, R Flaumenhaf, SM Parikh
J. Clin. Invest., 2018-03-05;0(0):.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Impaired angiopoietin/Tie2 signaling compromises Schlemm's canal integrity and induces glaucoma
Authors: J Kim, DY Park, H Bae, DY Park, D Kim, CK Lee, S Song, TY Chung, DH Lim, Y Kubota, YK Hong, Y He, HG Augustin, G Oliver, GY Koh
J. Clin. Invest., 2017-09-18;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Polydom Is an Extracellular Matrix Protein Involved in Lymphatic Vessel Remodeling
Authors: N Morooka, S Futaki, R Sato-Nishi, M Nishino, Y Totani, C Shimono, I Nakano, H Nakajima, N Mochizuki, K Sekiguchi
Circ. Res, 2017-02-08;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Shear stress-induced angiogenesis in mouse muscle is independent of the vasodilator mechanism and quickly reversible
Authors: S. Egginton, A. Hussain, J. Hall-Jones, B. Chaudhry, F. Syeda, K. E. Glen
Acta Physiol (Oxf)
Species: Mouse
Sample Types: Tissue Homogenates
Applications: Western Blot -
Sox7, Sox17, and Sox18 Cooperatively Regulate Vascular Development in the Mouse Retina.
Authors: Zhou Y, Williams J, Smallwood P, Nathans J
PLoS ONE, 2015-12-02;10(12):e0143650.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Endothelial destabilization by angiopoietin-2 via integrin beta1 activation.
Authors: Hakanpaa L, Sipila T, Leppanen V, Gautam P, Nurmi H, Jacquemet G, Eklund L, Ivaska J, Alitalo K, Saharinen P
Nat Commun, 2015-01-30;6(0):5962.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
COMP-angiopoietin-1 recovers molecular biomarkers of neuropathy and improves vascularisation in sciatic nerve of ob/ob mice.
Authors: Kosacka J, Nowicki M, Kloting N, Kern M, Stumvoll M, Bechmann I, Serke H, Bluher M
PLoS ONE, 2012-03-06;7(3):e32881.
Species: Mouse
Sample Types: Tissue Homogenates
Applications: Western Blot -
Ectopic expression of angiopoietin-1 promotes neuronal differentiation in neural progenitor cells through the Akt pathway.
Authors: Bai Y, Cui M, Meng Z, Shen L, He Q, Zhang X, Chen F, Xiao J
Biochem. Biophys. Res. Commun., 2008-11-24;378(2):296-301.
Species: Mouse
Sample Types: Cell Lysates
Applications: Western Blot -
A neurovascular niche for neurogenesis after stroke.
Authors: Ohab JJ, Fleming S, Blesch A, Carmichael ST
J. Neurosci., 2006-12-13;26(50):13007-16.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Immunotherapy of tumors with protein vaccine based on chicken homologous Tie-2.
Authors: Luo Y, Wen YJ, Ding ZY, Fu CH, Wu Y, Liu JY, Li Q, He QM, Zhao X, Jiang Y, Li J, Deng HX, Kang B, Mao YQ, Wei YQ
Clin. Cancer Res., 2006-03-15;12(6):1813-9.
Species: Chicken, Mouse
Sample Types: Recombinant Protein
Applications: Western Blot -
Angpt2 Induces Mesangial Cell Apoptosis through the MicroRNA-33-5p-SOCS5 Loop in Diabetic Nephropathy
Authors: Yi-Chun Tsai, Po-Lin Kuo, Wei-Wen Hung, Ling-Yu Wu, Ping-Hsun Wu, Wei-An Chang et al.
Molecular Therapy - Nucleic Acids
-
Angiopoietin receptor Tie2 is required for vein specification and maintenance via regulating COUP-TFII
Authors: Man Chu, Taotao Li, Bin Shen, Xudong Cao, Haoyu Zhong, Luqing Zhang et al.
eLife
-
Loss of flow responsive Tie1 results in Impaired Aortic valve remodeling
Authors: Xianghu Qu, Kate Violette, M. K. Sewell-Loftin, Jonathan Soslow, LeShana Saint-Jean, Robert B. Hinton et al.
Developmental Biology
-
Shear stress-induced angiogenesis in mouse muscle is independent of the vasodilator mechanism and quickly reversible
Authors: S. Egginton, A. Hussain, J. Hall-Jones, B. Chaudhry, F. Syeda, K. E. Glen
Acta Physiol (Oxf)
-
MicroRNA-711–Induced Downregulation of Angiopoietin-1 Mediates Neuronal Cell Death
Authors: Boris Sabirzhanov, Alan I. Faden, Taryn Aubrecht, Rebecca Henry, Ethan Glaser, Bogdan A. Stoica
Journal of Neurotrauma
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Mouse/Rat Tie-2 Antibody
Average Rating: 4 (Based on 1 Review)
Have you used Mouse/Rat Tie-2 Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:



