Mouse PD-1 Antibody Summary
Leu25-Gln167
Accession # Q02242
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
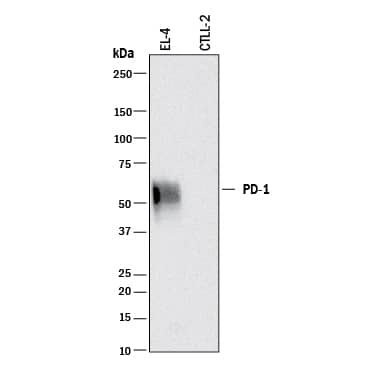
Detection of Mouse PD‑1 by Western Blot. Western blot shows lysates of EL-4 mouse lymphoblast cell line and CTLL-2 mouse cytotoxic T cell line (negative control). PVDF membrane was probed with 0.2 µg/mL of Goat Anti-Mouse PD-1 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF1021) followed by HRP-conjugated Anti-Goat IgG Secondary Antibody (Catalog # HAF017). A specific band was detected for PD-1 at approximately 55 kDa (as indicated). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 1.
 View Larger
View Larger
Detection of Mouse PD‑1 by Western Blot. Western blot shows lysates of 293T human embryonic kidney cell line mock transfected or transfected with mouse PD-1. PVDF membrane was probed with 0.2 µg/mL of Goat Anti-Mouse PD-1 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF1021) followed by HRP-conjugated Anti-Goat IgG Secondary Antibody (Catalog # HAF017). A specific band was detected for PD-1 at approximately 75 kDa (as indicated). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 1.
 View Larger
View Larger
PD‑1 in Mouse Thymus. PD‑1 was detected in perfusion fixed frozen sections of mouse thymus using 15 µg/mL Mouse PD‑1 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF1021) overnight at 4 °C. Tissue was stained (red) and counterstained (green). View our protocol for Fluorescent IHC Staining of Frozen Tissue Sections.
 View Larger
View Larger
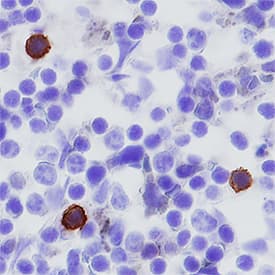
PD‑1 in Mouse Spleen. PD-1 was detected in perfusion fixed frozen sections of mouse spleen using Goat Anti-Mouse PD-1 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF1021) at 5 µg/mL overnight at 4 °C. Tissue was stained using the Anti-Goat HRP-DAB Cell & Tissue Staining Kit (brown; Catalog # CTS008) and counterstained with hematoxylin (blue). Specific staining was localized to cytoplasm in splenocytes. View our protocol for Chromogenic IHC Staining of Frozen Tissue Sections.
 View Larger
View Larger
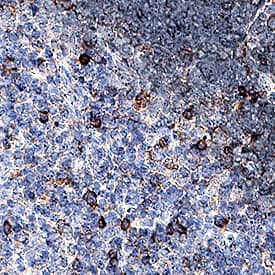
PD‑1 in Mouse Thymus. PD-1 was detected in perfusion fixed paraffin-embedded sections of mouse thymus using Goat Anti-Mouse PD-1 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF1021) at 1.7 µg/mL for 1 hour at room temperature followed by incubation with the Anti-Goat IgG VisUCyte™ HRP Polymer Antibody (Catalog # VC004). Before incubation with the primary antibody, tissue was subjected to heat-induced epitope retrieval using Antigen Retrieval Reagent-Basic (Catalog # CTS013). Tissue was stained using DAB (brown) and counterstained with hematoxylin (blue). Specific staining was localized to cell membranes. View our protocol for IHC Staining with VisUCyte HRP Polymer Detection Reagents.
 View Larger
View Larger
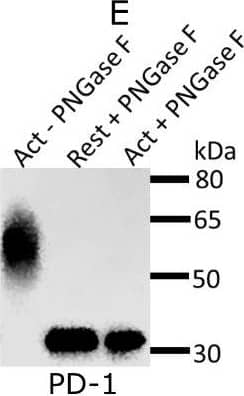
Detection of Mouse PD-1 by Western Blot Expression and glycosylation of Siglec-1 counter-receptors on resting and activated Tregs.(A) Eight Siglec-1 counter receptors were randomly selected for cytometry analysis. All experiments were performed three times and similar results were observed. (B) Normalized mRNA counts of Siglec-1 counter receptors were obtained from Treg RNA-Seq data. The result is visualized in a volcano plot. Only a small subset of counter-receptors showed strongly increased gene expression following Treg activation. The counter-receptors selected for flow cytometry analysis are highlighted in red. (C) Western blot showing that CD48 had a higher molecular weight in activated Tregs. (D) Western blot of PD-1 affinity-purified from resting and activated Tregs. PD 1 showed a dramatic decrease of molecular weight after PNGase F digestion (D), PD-1 from activated Tregs had higher molecular weight (E) and following sialidase treatment, PD-1 from resting and activated Tregs migrated at a slightly reduced molecular weight indicating the presence of sialic acids (F). Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35224210), licensed under a CC-BY license. Not internally tested by R&D Systems.
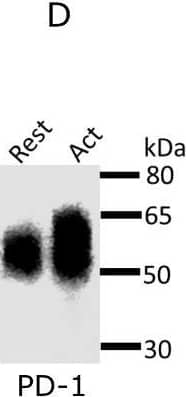 View Larger
View Larger
Detection of Mouse PD-1 by Western Blot Expression and glycosylation of Siglec-1 counter-receptors on resting and activated Tregs.(A) Eight Siglec-1 counter receptors were randomly selected for cytometry analysis. All experiments were performed three times and similar results were observed. (B) Normalized mRNA counts of Siglec-1 counter receptors were obtained from Treg RNA-Seq data. The result is visualized in a volcano plot. Only a small subset of counter-receptors showed strongly increased gene expression following Treg activation. The counter-receptors selected for flow cytometry analysis are highlighted in red. (C) Western blot showing that CD48 had a higher molecular weight in activated Tregs. (D) Western blot of PD-1 affinity-purified from resting and activated Tregs. PD 1 showed a dramatic decrease of molecular weight after PNGase F digestion (D), PD-1 from activated Tregs had higher molecular weight (E) and following sialidase treatment, PD-1 from resting and activated Tregs migrated at a slightly reduced molecular weight indicating the presence of sialic acids (F). Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35224210), licensed under a CC-BY license. Not internally tested by R&D Systems.
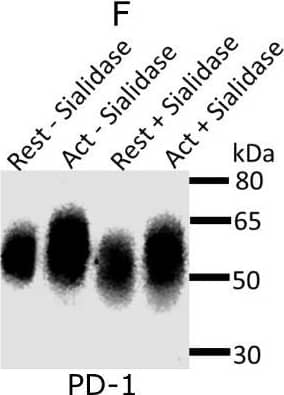 View Larger
View Larger
Detection of Mouse PD-1 by Western Blot Expression and glycosylation of Siglec-1 counter-receptors on resting and activated Tregs.(A) Eight Siglec-1 counter receptors were randomly selected for cytometry analysis. All experiments were performed three times and similar results were observed. (B) Normalized mRNA counts of Siglec-1 counter receptors were obtained from Treg RNA-Seq data. The result is visualized in a volcano plot. Only a small subset of counter-receptors showed strongly increased gene expression following Treg activation. The counter-receptors selected for flow cytometry analysis are highlighted in red. (C) Western blot showing that CD48 had a higher molecular weight in activated Tregs. (D) Western blot of PD-1 affinity-purified from resting and activated Tregs. PD 1 showed a dramatic decrease of molecular weight after PNGase F digestion (D), PD-1 from activated Tregs had higher molecular weight (E) and following sialidase treatment, PD-1 from resting and activated Tregs migrated at a slightly reduced molecular weight indicating the presence of sialic acids (F). Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35224210), licensed under a CC-BY license. Not internally tested by R&D Systems.
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: PD-1
Programmed Death-1 (PD-1) is type I transmembrane protein belonging to the CD28/CTLA-4 family of immunoreceptors that mediate signals for regulating immune responses (1). Other members of this family include CD28, CTLA-4, and ICOS (2-4). PD-1 is most closely related to CTLA-4 and shares approximately 24% amino acid (aa) sequence identity. The mouse PD-1 gene encodes a 288 aa protein with a putative 20 aa signal peptide, a 149 aa extracellular region with one immunoglobulin-like V-type domain, a 21 aa transmembrane domain, and a 98 aa cytoplasmic region. The cytoplasmic tail contains two tyrosine residues that form the immunoreceptor tyrosine-based inhibitory motif (ITIM) and immunoreceptor tyrosine-based switch motif (ITSM) that are important in mediating PD-1 signaling. Mouse and human PD-1 share approximately 69% aa sequence identity. Two B7 family proteins, PD-L1 (also called B7-H1) and PD-L2, have been identified as PD-1 ligands (5, 6). PD-1 is expressed on activated T cells, B cells, myeloid cells, and on a subset of thymocytes. PD-1 deficient mice have a defect in peripheral tolerance and spontaneously develop autoimmune diseases. Binding of PD-1 to PD-L1 or PD-L2 results in the inhibition of TCR-mediated proliferation and cytokine production as well as BCR-mediated signaling. PD-1 likely has an inhibitory role in regulating immune responses (1-4).
- Ishida, Y. et al. (1992) EMBO J. 11:3887.
- Sharpe, A.H. and G.J. Freeman (2002) Nat. Rev. Immunol. 2:116.
- Coyle, A. and J. Gutierrez-Ramos (2001) Nat. Immunol. 2:203.
- Nishimura, H. and T. Honjo (2001) Trends in Immunol. 22:265.
- Latchman Y. et al. (2001) Nature Immun. 2:261.
- Tamura, H. et al. (2001) Blood 97:1809.
Product Datasheets
Citations for Mouse PD-1 Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
25
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Highly multiplexed immunofluorescence imaging of human tissues and tumors using t-CyCIF and conventional optical microscopes.
Authors: Lin J. R, Izar B, et al.
Elife
-
Transgenic viral expression of PH-20, IL-12, and sPD1-Fc enhances immune cell infiltration and anti-tumor efficacy of an oncolytic virus
Authors: Soon-Oh Hong, Joonsung Kim, Sungmin Lee, Jaeil Shin, Hwanjun Choi, Eunjin Lee et al.
Mol Ther Oncolytics
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Orthogonal cytokine engineering enables novel synthetic effector states escaping canonical exhaustion in tumor-rejecting CD8+ T cells
Authors: Corria-Osorio J, Carmona SJ, Stefanidis E, Andreatta M, Ortiz-Miranda Y, Muller T, Rota IA, Crespo I, Seijo B, Castro W, Jimenez-Luna C, Scarpellino L, Ronet C, Spill A, Lanitis E, Romero P, Luther SA, Irving M, Coukos G
Nat Immunol. 2023; 24(5): 869–883.
Species: Mouse
Sample Types: Cell Culture Supernates
Applications: ELISA Capture -
Mice transgenic for human CTLA4-CD28 fusion gene show proliferation and transformation of ATLL-like and AITL-like T cells
Authors: Gyu Jin Lee, Yukyung Jun, Yoon Kyung Jeon, Daekee Lee, Sanghyuk Lee, Jaesang Kim
OncoImmunology
-
CD8+ T cells induce interferon-responsive oligodendrocytes and microglia in white matter aging
Authors: T Kaya, N Mattugini, L Liu, H Ji, L Cantuti-Ca, J Wu, M Schifferer, J Groh, R Martini, S Besson-Gir, S Kaji, A Liesz, O Gokce, M Simons
Nature Neuroscience, 2022-10-24;25(11):1446-1457.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
PD-1-cis IL-2R agonism yields better effectors from stem-like CD8+ T cells
Authors: L Codarri De, V Nicolini, M Hashimoto, M Karagianni, PC Schwalie, L Lauener, EM Varypataki, M Richard, E Bommer, J Sam, S Joller, M Perro, F Cremasco, L Kunz, E Yanguez, T Hüsser, R Schlenker, M Mariani, V Tosevski, S Herter, M Bacac, I Waldhauer, S Colombetti, X Gueripel, S Wullschleg, M Tichet, D Hanahan, HT Kissick, S Leclair, A Freimoser-, S Seeber, V Teichgräbe, R Ahmed, C Klein, P Umaña
Nature, 2022-09-28;610(7930):161-172.
Species: Xenograft
Sample Types: Whole Tissue
Applications: IHC -
Distinct antibody clones detect PD-1 checkpoint expression and block PD-L1 interactions on live murine melanoma cells
Authors: C Martins, M Silva, E Rasbach, P Singh, Y Itoh, JB Williams, E Statham, A Meurer, DV Martinez, A Brandenbur, MV Heppt, SR Barthel, T Schatton
Scientific Reports, 2022-07-21;12(1):12491.
Species: Mouse
Sample Types: Cell Lysates
Applications: Western Blot -
AXL Inhibition in Macrophages Stimulates Host-versus-Leukemia Immunity and Eradicates Naïve and Treatment-Resistant Leukemia
Authors: Irene Tirado-Gonzalez, Arnaud Descot, Devona Soetopo, Aleksandra Nevmerzhitskaya, Alexander Schäffer, Ivan-Maximilano Kur et al.
Cancer Discovery
-
Activation of regulatory T cells triggers specific changes in glycosylation associated with Siglec-1-dependent inflammatory responses
Authors: Gang Wu, Gavuthami Murugesan, Manjula Nagala, Alex McCraw, Stuart M. Haslam, Anne Dell et al.
Wellcome Open Research
Species: Mouse
Sample Types: Cell Lysates
Applications: Western Blot -
Eliminating mesothelioma by AAV-vectored, PD1-based vaccination in the tumor microenvironment
Authors: Zhiwu Tan, Mei Sum Chiu, Chi Wing Yan, Kwan Man, Zhiwei Chen
Molecular Therapy - Oncolytics
-
Auto-aggressive CXCR6+ CD8 T cells cause liver immune pathology in NASH
Authors: M Dudek, D Pfister, S Donakonda, P Filpe, A Schneider, M Laschinger, D Hartmann, N Hüser, P Meiser, F Bayerl, D Inverso, J Wigger, M Sebode, R Öllinger, R Rad, S Hegenbarth, M Anton, A Guillot, A Bowman, D Heide, F Müller, P Ramadori, V Leone, C Garcia-Cac, T Gruber, G Seifert, AM Kabat, JP Malm, S Reider, M Effenberge, S Roth, AT Billeter, B Müller-Sti, EJ Pearce, F Koch-Nolte, R Käser, H Tilg, R Thimme, T Böttler, F Tacke, JF Dufour, D Haller, PJ Murray, R Heeren, D Zehn, JP Böttcher, M Heikenwäld, PA Knolle
Nature, 2021-03-24;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Inhibitory Receptor Trap: A Platform for Discovery of Inhibitory Receptors That Utilize Inositol Lipid and Phosphotyrosine Phosphatase Effectors
Authors: Bergren W. Crute, Rachel Sheraden, Vanessa L. Ott, Isaac T. W. Harley, Andrew Getahun, John C. Cambier
Frontiers in Immunology
-
Inhibition of TRPV1 by SHP-1 in nociceptive primary sensory neurons is critical in PD-L1 analgesia
Authors: Ben-Long Liu, Qi-Lai Cao, Xin Zhao, Hui-Zhu Liu, Yu-Qiu Zhang
JCI Insight
-
Angioimmunoblastic T-cell lymphoma-like lymphadenopathy in mice transgenic for human RHOA with p.Gly17Val mutation
Authors: Gyu Jin Lee, Yukyung Jun, Hae Yong Yoo, Yoon Kyung Jeon, Daekee Lee, Sanghyuk Lee et al.
OncoImmunology
-
Anti–PD-1 Induces M1 Polarization in the Glioma Microenvironment and Exerts Therapeutic Efficacy in the Absence of CD8 Cytotoxic T Cells
Authors: Ganesh Rao, Khatri Latha, Martina Ott, Aria Sabbagh, Anantha Marisetty, Xiaoyang Ling et al.
Clinical Cancer Research
-
CD47 prevents the elimination of diseased fibroblasts in scleroderma
Authors: T Lerbs, L Cui, ME King, T Chai, C Muscat, L Chung, R Brown, K Rieger, T Shibata, G Wernig
JCI Insight, 2020-08-20;5(16):.
Species: Mouse
Sample Types: Whole Cells, Whole Tissue
Applications: ICC, IHC -
HIF-1alpha and HIF-2alpha differently regulate tumour development and inflammation of clear cell renal cell carcinoma in mice
Authors: R Hoefflin, S Harlander, S Schäfer, P Metzger, F Kuo, D Schönenber, M Adlesic, A Peighambar, P Seidel, CY Chen, M Consenza-C, A Jud, B Lahrmann, N Grabe, D Heide, FM Uhl, TA Chan, J Duyster, R Zeiser, C Schell, M Heikenwald, O Schilling, AA Hakimi, M Boerries, IJ Frew
Nat Commun, 2020-08-17;11(1):4111.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Bcl-6-directed follicular helper T cells promote vascular inflammatory injury in diabetic retinopathy
Authors: Yan Liu, Ziqi Yang, Peilong Lai, Zijing Huang, Xiaowei Sun, Tian Zhou et al.
Theranostics
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
IL-17C-mediated innate inflammation decreases the response to PD-1 blockade in a model of Kras-driven lung cancer
Authors: F Ritzmann, C Jungnickel, G Vella, A Kamyschnik, C Herr, D Li, MM Menger, A Angenendt, M Hoth, A Lis, R Bals, C Beisswenge
Sci Rep, 2019-07-17;9(1):10353.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Expression of lymphocyte-activating gene 3 and T-cell immunoreceptor with immunoglobulin and ITIM domains in cutaneous melanoma and their correlation with programmed cell death 1 expression in tumor-infiltrating lymphocytes
Authors: WJ Lee, YJ Lee, ME Choi, KA Yun, CH Won, MW Lee, JH Choi, SE Chang
J. Am. Acad. Dermatol., 2019-03-14;81(1):219-227.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
CD8?? intraepithelial lymphocytes arise from two main thymic precursors
Authors: R Ruscher, RL Kummer, YJ Lee, SC Jameson, KA Hogquist
Nat. Immunol., 2017-05-22;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
The development of endometrial hyperplasia in aged PD-1-deficient female mice
Authors: Guoning Guo, Hong Li, Dayan Cao, Yongwen Chen
Diagnostic Pathology
-
Anti-programmed cell death 1 antibody reduces CD4+PD-1+ T cells and relieves the lupus-like nephritis of NZB/W F1 mice.
Authors: Kasagi S, Kawano S, Okazaki T, Honjo T, Morinobu A, Hatachi S, Shimatani K, Tanaka Y, Minato N, Kumagai S
J. Immunol., 2010-02-05;184(5):2337-47.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Depletion of the programmed death-1 receptor completely reverses established clonal anergy in CD4(+) T lymphocytes via an interleukin-2-dependent mechanism.
Authors: Bishop KD, Harris JE, Mordes JP, Greiner DL, Rossini AA, Czech MP, Phillips NE
Cell. Immunol., 2009-02-23;256(1):86-91.
Species: Mouse
Sample Types: Cell Lysates
Applications: Western Blot -
IL-10 release upon PD-1 blockade sustains immunosuppression in ovarian cancer
Authors: P Lamichhane, L Karyampudi, B Shreeder, J Krempski, D Bahr, J Daum, KR Kalli, EL Goode, MS Block, MJ Cannon, KL Knutson
Cancer Res., 2017-10-09;0(0):.
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Mouse PD-1 Antibody
Average Rating: 4.5 (Based on 4 Reviews)
Have you used Mouse PD-1 Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:

