Mouse Cathepsin B Antibody Summary
His18-Phe339
Accession # P10605
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
Cathepsin B in Mouse Thymus. Cathepsin B was detected in perfusion fixed frozen sections of mouse thymus using 5 µg/mL Goat Anti-Mouse Cathepsin B Antigen Affinity-purified Polyclonal Antibody (Catalog # AF965) overnight at 4 °C. Tissue was stained with the Anti-Goat HRP-DAB Cell & Tissue Staining Kit (brown; Catalog # CTS008) and counterstained with hematoxylin (blue). View our protocol for Chromogenic IHC Staining of Frozen Tissue Sections.
 View Larger
View Larger
Detection of Mouse Cathepsin B by Immunocytochemistry/ Immunofluorescence EMT in BSA-induced damaged tubule was associated with increased levels of DPP-4, integrin beta 1 and CAV1; TENE treatment ameliorated these alterations. (a–e) Multiplex immunofluorescence microscopy analysis of the EMT program and association with CAV1. Formaldehyde-fixed, paraffin-embedded (FFPE) kidney samples were labeled with epithelial markers for E-cadherin, alpha SMA and CAV1. An immunofluorescence analysis was performed by confocal microscopy. (d) The enlarged image of the inset shown in (c). The alpha SMA-positive damaged tubular cells were surrounded by alpha SMA-positive interstitial cells (f–j). Multiplex immunofluorescence was performed to analyze the crosstalk among DPP-4, integrin beta 1 and CAV1 in the BSA-injected diabetic mice. (i) The enlarged image of the inset shown in (h). DPP-4, integrin beta 1, and CAV1 were localized at the same location (likely the luminal side of the proximal tubule). The crosstalk occurred more frequently in the damaged tubular cells. Representative images from n = 7 in each group are shown. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/31101909), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Cathepsin B by Immunohistochemistry BSA-injected diabetic mice exhibited high tubular levels of DPP-4, CAV1 and EMT program; TENE treatment ameliorated these alterations. Immunohistochemical analysis of (a–d) DPP-4, (e–h) CAV1, (i–l) snail and (m–p) AQP-1 from the BSA-injected control or diabetic mice with or without the TENE treatment. Scale bar, 50 μm. Representative images from n = 7 in each group are shown. Each group was analyzed with an unpaired two-tailed t-test. *P < 0.05, **P < 0.01. Data are presented as mean ± s.e.m (q–t). Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/31101909), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Cathepsin B by Immunocytochemistry/ Immunofluorescence EMT in BSA-induced damaged tubule was associated with increased levels of DPP-4, integrin beta 1 and CAV1; TENE treatment ameliorated these alterations. (a–e) Multiplex immunofluorescence microscopy analysis of the EMT program and association with CAV1. Formaldehyde-fixed, paraffin-embedded (FFPE) kidney samples were labeled with epithelial markers for E-cadherin, alpha SMA and CAV1. An immunofluorescence analysis was performed by confocal microscopy. (d) The enlarged image of the inset shown in (c). The alpha SMA-positive damaged tubular cells were surrounded by alpha SMA-positive interstitial cells (f–j). Multiplex immunofluorescence was performed to analyze the crosstalk among DPP-4, integrin beta 1 and CAV1 in the BSA-injected diabetic mice. (i) The enlarged image of the inset shown in (h). DPP-4, integrin beta 1, and CAV1 were localized at the same location (likely the luminal side of the proximal tubule). The crosstalk occurred more frequently in the damaged tubular cells. Representative images from n = 7 in each group are shown. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/31101909), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Cathepsin B by Immunocytochemistry/ Immunofluorescence Alteration of the size of lysosomes and the expression of LAMP-1 in in Rab7 delta pan pancreatic acinar cells. (a–d) Immunofluorescence images of wild (a,c) and Rab7 delta pan (b,d) pancreases stained with anti-LAMP1(a,b) or anti-cathepsin B (c,d) antibodies (red). DAPI was used for nuclear staining (blue). Bars: 20 µm. (e) Quantification of the positive signals in immunofluorescence images of LAMP-1 (left panel) and cathepsin B (right panel). *P < 0.05. (f) WB of LAMP-1 using total pancreas homogenate of wild and Rab7 delta pan mice. An antibody against LAMP-1 N-terminal (top panel) and an antibody against LAMP-1 C-terminal (middle panel) were utilized. Anti-LAMP-1 N-terminal antibody revealed the shifting of intense bands to a lower position (top panel, arrow head) than that of full-length LAMP-1 (120 kDa) in Rab7 delta pan pancreas. In contrast, anti-LAMP-1 C-terminal antibody revealed bands at 120 kDa only in both wild and Rab7 delta pan pancreas (middle panel). beta -actin was used as an internal loading control (bottom panel). Image collected and cropped by CiteAb from the following open publication (https://www.nature.com/articles/s41598-017-02988-3), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Cathepsin B by Immunocytochemistry/ Immunofluorescence Alteration of the size of lysosomes and the expression of LAMP-1 in in Rab7 delta pan pancreatic acinar cells. (a–d) Immunofluorescence images of wild (a,c) and Rab7 delta pan (b,d) pancreases stained with anti-LAMP1(a,b) or anti-cathepsin B (c,d) antibodies (red). DAPI was used for nuclear staining (blue). Bars: 20 µm. (e) Quantification of the positive signals in immunofluorescence images of LAMP-1 (left panel) and cathepsin B (right panel). *P < 0.05. (f) WB of LAMP-1 using total pancreas homogenate of wild and Rab7 delta pan mice. An antibody against LAMP-1 N-terminal (top panel) and an antibody against LAMP-1 C-terminal (middle panel) were utilized. Anti-LAMP-1 N-terminal antibody revealed the shifting of intense bands to a lower position (top panel, arrow head) than that of full-length LAMP-1 (120 kDa) in Rab7 delta pan pancreas. In contrast, anti-LAMP-1 C-terminal antibody revealed bands at 120 kDa only in both wild and Rab7 delta pan pancreas (middle panel). beta -actin was used as an internal loading control (bottom panel). Image collected and cropped by CiteAb from the following open publication (https://www.nature.com/articles/s41598-017-02988-3), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Cathepsin B by Immunocytochemistry/ Immunofluorescence EMT in BSA-induced damaged tubule was associated with increased levels of DPP-4, integrin beta 1 and CAV1; TENE treatment ameliorated these alterations. (a–e) Multiplex immunofluorescence microscopy analysis of the EMT program and association with CAV1. Formaldehyde-fixed, paraffin-embedded (FFPE) kidney samples were labeled with epithelial markers for E-cadherin, alpha SMA and CAV1. An immunofluorescence analysis was performed by confocal microscopy. (d) The enlarged image of the inset shown in (c). The alpha SMA-positive damaged tubular cells were surrounded by alpha SMA-positive interstitial cells (f–j). Multiplex immunofluorescence was performed to analyze the crosstalk among DPP-4, integrin beta 1 and CAV1 in the BSA-injected diabetic mice. (i) The enlarged image of the inset shown in (h). DPP-4, integrin beta 1, and CAV1 were localized at the same location (likely the luminal side of the proximal tubule). The crosstalk occurred more frequently in the damaged tubular cells. Representative images from n = 7 in each group are shown. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/31101909), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Cathepsin B by Immunohistochemistry BSA-injected diabetic mice exhibited high tubular levels of DPP-4, CAV1 and EMT program; TENE treatment ameliorated these alterations. Immunohistochemical analysis of (a–d) DPP-4, (e–h) CAV1, (i–l) snail and (m–p) AQP-1 from the BSA-injected control or diabetic mice with or without the TENE treatment. Scale bar, 50 μm. Representative images from n = 7 in each group are shown. Each group was analyzed with an unpaired two-tailed t-test. *P < 0.05, **P < 0.01. Data are presented as mean ± s.e.m (q–t). Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/31101909), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Cathepsin B by Western Blot TENE treatment suppressed the crosstalk among DPP-4, integrin beta 1 and CAV1 via inhibition of TGF-beta /smad3 signaling pathway in vitro. Duolink in situ analysis of (a-c) DPP-4/integrin beta 1, (d–f) DPP-4/CAV1 and (g–i) integrin beta 1/CAV1 in HK-2 cells with or without TGF-beta 1 (10 ng/ml) was performed by confocal microscopy (×1260). Scale bar: 50 μm in each panel. (j) Representative western blot analysis. As a densitometric analysis, each protein level was normalized with actin. n = 6 per group were analyzed. (k–n) Duolink in situ analysis of integrin beta 1/CAV1 in DPP-4 overexpressed HK-2 cells with or without TENE and SIS3. (o) Immunoprecipitation analysis revealed TGF-beta treatment increased crosstalk among DPP-4, integrin beta 1 (ITG beta 1) and CAV1. (p) Immunoprecipitation assay revealed TGF-beta neutralization suppressed crosstalk among DPP-4, integrin beta 1 and CAV1 induced by DPP-4 overexpression. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/31101909), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Cathepsin B by Immunohistochemistry BSA-injected diabetic mice exhibited high tubular levels of DPP-4, CAV1 and EMT program; TENE treatment ameliorated these alterations. Immunohistochemical analysis of (a–d) DPP-4, (e–h) CAV1, (i–l) snail and (m–p) AQP-1 from the BSA-injected control or diabetic mice with or without the TENE treatment. Scale bar, 50 μm. Representative images from n = 7 in each group are shown. Each group was analyzed with an unpaired two-tailed t-test. *P < 0.05, **P < 0.01. Data are presented as mean ± s.e.m (q–t). Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/31101909), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Cathepsin B by Western Blot TENE treatment suppressed the crosstalk among DPP-4, integrin beta 1 and CAV1 via inhibition of TGF-beta /smad3 signaling pathway in vitro. Duolink in situ analysis of (a-c) DPP-4/integrin beta 1, (d–f) DPP-4/CAV1 and (g–i) integrin beta 1/CAV1 in HK-2 cells with or without TGF-beta 1 (10 ng/ml) was performed by confocal microscopy (×1260). Scale bar: 50 μm in each panel. (j) Representative western blot analysis. As a densitometric analysis, each protein level was normalized with actin. n = 6 per group were analyzed. (k–n) Duolink in situ analysis of integrin beta 1/CAV1 in DPP-4 overexpressed HK-2 cells with or without TENE and SIS3. (o) Immunoprecipitation analysis revealed TGF-beta treatment increased crosstalk among DPP-4, integrin beta 1 (ITG beta 1) and CAV1. (p) Immunoprecipitation assay revealed TGF-beta neutralization suppressed crosstalk among DPP-4, integrin beta 1 and CAV1 induced by DPP-4 overexpression. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/31101909), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Cathepsin B by Western Blot TENE treatment suppressed the crosstalk among DPP-4, integrin beta 1 and CAV1 via inhibition of TGF-beta /smad3 signaling pathway in vitro. Duolink in situ analysis of (a-c) DPP-4/integrin beta 1, (d–f) DPP-4/CAV1 and (g–i) integrin beta 1/CAV1 in HK-2 cells with or without TGF-beta 1 (10 ng/ml) was performed by confocal microscopy (×1260). Scale bar: 50 μm in each panel. (j) Representative western blot analysis. As a densitometric analysis, each protein level was normalized with actin. n = 6 per group were analyzed. (k–n) Duolink in situ analysis of integrin beta 1/CAV1 in DPP-4 overexpressed HK-2 cells with or without TENE and SIS3. (o) Immunoprecipitation analysis revealed TGF-beta treatment increased crosstalk among DPP-4, integrin beta 1 (ITG beta 1) and CAV1. (p) Immunoprecipitation assay revealed TGF-beta neutralization suppressed crosstalk among DPP-4, integrin beta 1 and CAV1 induced by DPP-4 overexpression. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/31101909), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Cathepsin B by Immunohistochemistry BSA-injected diabetic mice exhibited high tubular levels of DPP-4, CAV1 and EMT program; TENE treatment ameliorated these alterations. Immunohistochemical analysis of (a–d) DPP-4, (e–h) CAV1, (i–l) snail and (m–p) AQP-1 from the BSA-injected control or diabetic mice with or without the TENE treatment. Scale bar, 50 μm. Representative images from n = 7 in each group are shown. Each group was analyzed with an unpaired two-tailed t-test. *P < 0.05, **P < 0.01. Data are presented as mean ± s.e.m (q–t). Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/31101909), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Cathepsin B by Immunocytochemistry/ Immunofluorescence EMT in BSA-induced damaged tubule was associated with increased levels of DPP-4, integrin beta 1 and CAV1; TENE treatment ameliorated these alterations. (a–e) Multiplex immunofluorescence microscopy analysis of the EMT program and association with CAV1. Formaldehyde-fixed, paraffin-embedded (FFPE) kidney samples were labeled with epithelial markers for E-cadherin, alpha SMA and CAV1. An immunofluorescence analysis was performed by confocal microscopy. (d) The enlarged image of the inset shown in (c). The alpha SMA-positive damaged tubular cells were surrounded by alpha SMA-positive interstitial cells (f–j). Multiplex immunofluorescence was performed to analyze the crosstalk among DPP-4, integrin beta 1 and CAV1 in the BSA-injected diabetic mice. (i) The enlarged image of the inset shown in (h). DPP-4, integrin beta 1, and CAV1 were localized at the same location (likely the luminal side of the proximal tubule). The crosstalk occurred more frequently in the damaged tubular cells. Representative images from n = 7 in each group are shown. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/31101909), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Cathepsin B by Immunocytochemistry/ Immunofluorescence EMT in BSA-induced damaged tubule was associated with increased levels of DPP-4, integrin beta 1 and CAV1; TENE treatment ameliorated these alterations. (a–e) Multiplex immunofluorescence microscopy analysis of the EMT program and association with CAV1. Formaldehyde-fixed, paraffin-embedded (FFPE) kidney samples were labeled with epithelial markers for E-cadherin, alpha SMA and CAV1. An immunofluorescence analysis was performed by confocal microscopy. (d) The enlarged image of the inset shown in (c). The alpha SMA-positive damaged tubular cells were surrounded by alpha SMA-positive interstitial cells (f–j). Multiplex immunofluorescence was performed to analyze the crosstalk among DPP-4, integrin beta 1 and CAV1 in the BSA-injected diabetic mice. (i) The enlarged image of the inset shown in (h). DPP-4, integrin beta 1, and CAV1 were localized at the same location (likely the luminal side of the proximal tubule). The crosstalk occurred more frequently in the damaged tubular cells. Representative images from n = 7 in each group are shown. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/31101909), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Cathepsin B by Immunocytochemistry/ Immunofluorescence Myc induces cathepsin L expression in beta-cells of pancreatic Islets.(A) Immunohistochemical analyses for CTS B, C, L or S expression (all in red) in combination with staining for the pan-leukocyte marker CD45 (green) in pancreatic islet tumors from the MycERTAM;Bcl-xL animals. Pancreata were harvested from the MycERTAM;Bcl-xL mice treated for 7 d with TAM (Myc-On, 7 days) or control vehicle in place of TAM (Myc-OFF). The islet area is indicated by dotted line. The asterisks indicate the area of tumor represented in the insets. The panels are representatives of at least three animals assayed at each data point, all immunohistochemical analyses were done in duplicate; eight randomized fields per analysis were examined. Scale bars, 100μm. (B) Immunohistochemical analysis for cathepsin L expression in beta-cells of pancreatic islets from MycERTAM;Bcl-xL animals identified by insulin expression. Pancreata were collected from the animals described above. Scale bars represent 25μm. The panels are representatives of three animals assayed at each data point, all immunohistochemical analyses were done in duplicate; ten randomized fields per analysis were examined. Image collected and cropped by CiteAb from the following open publication (https://dx.plos.org/10.1371/journal.pone.0120348), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Cathepsin B by Immunocytochemistry/ Immunofluorescence Myc induces cathepsin L expression in beta-cells of pancreatic Islets.(A) Immunohistochemical analyses for CTS B, C, L or S expression (all in red) in combination with staining for the pan-leukocyte marker CD45 (green) in pancreatic islet tumors from the MycERTAM;Bcl-xL animals. Pancreata were harvested from the MycERTAM;Bcl-xL mice treated for 7 d with TAM (Myc-On, 7 days) or control vehicle in place of TAM (Myc-OFF). The islet area is indicated by dotted line. The asterisks indicate the area of tumor represented in the insets. The panels are representatives of at least three animals assayed at each data point, all immunohistochemical analyses were done in duplicate; eight randomized fields per analysis were examined. Scale bars, 100μm. (B) Immunohistochemical analysis for cathepsin L expression in beta-cells of pancreatic islets from MycERTAM;Bcl-xL animals identified by insulin expression. Pancreata were collected from the animals described above. Scale bars represent 25μm. The panels are representatives of three animals assayed at each data point, all immunohistochemical analyses were done in duplicate; ten randomized fields per analysis were examined. Image collected and cropped by CiteAb from the following open publication (https://dx.plos.org/10.1371/journal.pone.0120348), licensed under a CC-BY license. Not internally tested by R&D Systems.
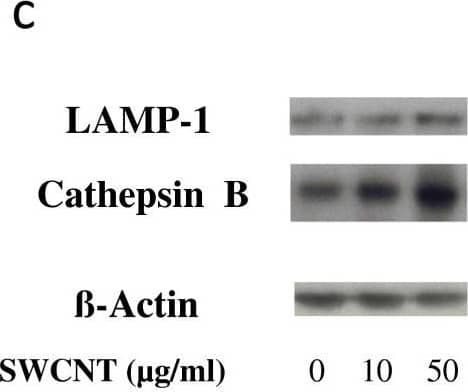 View Larger
View Larger
Detection of Cathepsin B by Western Blot Evaluation of lysosomal activation in SWCNT-exposed cells. Panel a: Epi-fluorescence images of macrophages unexposed (upper line) or exposed (lower line) to 50 μg/ml SWCNT for 24 hours stained with Acridine Orange (for lysosomes, in red) and DAPI (RNA/DNA, in green). Merge: combination of Acridine Orange and DAPI staining (overlapping appeared yellow). Panel b: Cathepsin activity in macrophages exposed to SWCNT for 3 hours. *: p < 0.001 between groups. Panel c: Western Blot images of LAMP-1 (120 kDa) and Cathepsin B (37 kDa) expression in macrophages exposed for 24 hours to SWCNT. beta -Actin is given as internal standard. Panel d: quantification of LAMP-1 and Cathepsin B expression, normalized to beta -Actin expression. *: p < 0.05 between groups. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/23800198), licensed under a CC-BY license. Not internally tested by R&D Systems.
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: Cathepsin B
Cathepsin B is the first described member of the family of lysosomal cysteine proteases (1). Cathepsin B possesses both endopeptidase and exopeptidase activities, in the latter case acting as a peptidyl-dipeptidase. It is known to process a number of proteins, including pro and active caspases, prorenin and secretory leucoprotease inhibitor (SLPI) (2‑4). Therefore, Cathepsin B may play a role in activation and inactivation of caspases, activation of renin and inactivation of SLPI, the key steps in apoptosis, angiotensin production, and progression of emphysema, respectively. Because of its increased levels and redistribution in human and animal tumors, Cathepsin B may also have a role in invasion and metastasis (5). In addition to the lysosome, Cathepsin B can be secreted or associated with plasma membrane, cytoplasm, and nucleus. It is synthesized as a preproenzyme. Following removal of the signal peptide, the inactive proenzyme undergoes further modifications including removal of the pro region to result in the active enzyme (5).
- Mort, J.S. (2004) in Handbook of Proteolytic Enzymes (Barrett, A.J. et al. eds.) p. 1079, Academic Press, San Diego.
- Vancompernolle, K. et al. (1998) FEBS Lett. 438:150.
- Jutras, I. and T.L. Reudelhuber (1998) FEBS Lett. 443:48.
- Taggart, C.C. et al. (2001) J. Biol. Chem. 276:33345.
- Berquin, I.M. and B.F. Sloane (1996) Adv. Exp. Med. Biol. 389:281.
Product Datasheets
Citations for Mouse Cathepsin B Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
51
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Degradation of dendritic cargos requires Rab7-dependent transport to somatic lysosomes
Authors: Chan Choo Yap, Laura Digilio, Lloyd P. McMahon, A. Denise R. Garcia, Bettina Winckler
Journal of Cell Biology
-
Retinoic acid regulates cell-shape and -death of E-FABP (FABP5)-immunoreactive septoclasts in the growth plate cartilage of mice
Authors: Yasuhiko Bando, Miyuki Yamamoto, Koji Sakiyama, Hide Sakashita, Fuyoko Taira, Genki Miyake et al.
Histochemistry and Cell Biology
-
Cathepsin B modulates lysosomal biogenesis and host defense against Francisella novicida infection
J Exp Med, 2016-08-22;0(0):.
-
Loss of TMEM106B and PGRN leads to severe lysosomal abnormalities and neurodegeneration in mice
Authors: Feng T, Mai S, Roscoe JM et al.
EMBO Rep.
-
Loss of TMEM106B Ameliorates Lysosomal and Frontotemporal Dementia-Related Phenotypes in Progranulin-Deficient Mice
Authors: Zoe A. Klein, Hideyuki Takahashi, Mengxiao Ma, Massimiliano Stagi, Melissa Zhou, TuKiet T. Lam et al.
Neuron
-
A multifaceted role of progranulin in regulating amyloid-beta dynamics and responses
Authors: Du H, Wong MY, Zhang T et al.
Life science alliance
-
LC3B phosphorylation regulates FYCO1 binding and directional transport of autophagosomes
Authors: Torres JLN, Shanahan SL, Chassefeyre R, Bueno SL
Curr Biol
-
Development of Activity-Based Probes for Cathepsin X
Authors: Margot G. Paulick, Matthew Bogyo
ACS Chemical Biology
-
Microglial P2X4 receptors promote ApoE degradation and contribute to memory deficits in Alzheimer’s disease
Authors: Jennifer Hua, Elvira Garcia de Paco, Nathalie Linck, Tangui Maurice, Catherine Desrumaux, Bénédicte Manoury et al.
Cellular and Molecular Life Sciences
-
Dipeptidyl peptidase-4 plays a pathogenic role in BSA-induced kidney injury in diabetic mice
Authors: Y Takagaki, S Shi, M Katoh, M Kitada, K Kanasaki, D Koya
Sci Rep, 2019-05-17;9(1):7519.
-
Synaptojanin1 Modifies Endolysosomal Parameters in Cultured Ventral Midbrain Neurons
Authors: Xinyu Zhu, Sanjana Surya Prakash, Geoffrey McAuliffe, Ping-Yue Pan
eNeuro
-
Lipid-mediated motor-adaptor sequestration impairs axonal lysosome delivery leading to autophagic stress and dystrophy in Niemann-Pick type C
Authors: Roney JC, Li S, Farfel-Becker T et al.
Developmental cell
-
Expression and enhancement of FABP4 in septoclasts of the growth plate in FABP5-deficient mouse tibiae
Authors: Yasuhiko Bando, Nobuko Tokuda, Yudai Ogasawara, Go Onozawa, Arata Nagasaka, Koji Sakiyama et al.
Histochemistry and Cell Biology
-
TMEM106B deficiency impairs cerebellar myelination and synaptic integrity with Purkinje cell loss
Authors: T Feng, L Luan, II Katz, M Ullah, VM Van Deerli, JQ Trojanowsk, EB Lee, F Hu
Acta neuropathologica communications, 2022-03-14;10(1):33.
-
LAMTOR1 inhibition of TRPML1‐dependent lysosomal calcium release regulates dendritic lysosome trafficking and hippocampal neuronal function
Authors: Jiandong Sun, Yan Liu, Xiaoning Hao, Weiju Lin, Wenyue Su, Emerald Chiang et al.
The EMBO Journal
-
The V-ATPase complex component RNAseK is required for lysosomal hydrolase delivery and autophagosome degradation
Authors: Makar, AN;Boraman, A;Mosen, P;Simpson, JE;Marques, J;Michelberger, T;Aitken, S;Wheeler, AP;Winter, D;von Kriegsheim, A;Gammoh, N;
Nature communications
Species: Mouse
Sample Types: Cell Lysates
Applications: Western Blot -
Reduced progranulin increases tau and ?-synuclein inclusions and alters mouse tauopathy phenotypes via glucocerebrosidase
Authors: Takahashi, H;Bhagwagar, S;Nies, SH;Ye, H;Han, X;Chiasseu, MT;Wang, G;Mackenzie, IR;Strittmatter, SM;
Nature communications
Species: Mouse
Sample Types: Tissue Homogenates
Applications: Western Blot -
Investigations on Primary Cilia of Nthy-ori 3-1 Cells upon Cysteine Cathepsin Inhibition or Thyrotropin Stimulation
Authors: Do?ru, AG;Rehders, M;Brix, K;
International journal of molecular sciences
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
TMEM106B regulates microglial proliferation and survival in response to demyelination
Authors: Zhang, T;Pang, W;Feng, T;Guo, J;Wu, K;Nunez Santos, M;Arthanarisami, A;Nana, AL;Nguyen, Q;Kim, PJ;Jankowsky, JL;Seeley, WW;Hu, F;
Science advances
Species: Mouse
Sample Types: Cell Lysates
Applications: Western Blot -
Sphingomyelin 16:0 is a therapeutic target for neuronal death in acid sphingomyelinase deficiency
Authors: Á Gaudioso, X Jiang, J Casas, EH Schuchman, MD Ledesma
Cell Death & Disease, 2023-04-06;14(4):248.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
Definition of the contribution of an Osteopontin-producing CD11c+ microglial subset to Alzheimer's disease
Authors: Y Qiu, X Shen, O Ravid, D Atrakchi, D Rand, AE Wight, HJ Kim, S Liraz-Zalt, I Cooper, M Schnaider, H Cantor
Proceedings of the National Academy of Sciences of the United States of America, 2023-02-02;120(6):e2218915120.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Liquid-liquid phase separation facilitates the biogenesis of secretory storage granules
Authors: Parchure A, Tian M, Stalder D et al.
The Journal of cell biology
-
Direct control of lysosomal catabolic activity by mTORC1 through regulation of V-ATPase assembly
Authors: E Ratto, SR Chowdhury, NS Siefert, M Schneider, M Wittmann, D Helm, W Palm
Nature Communications, 2022-08-17;13(1):4848.
Species: Mouse
Sample Types: Cell Lysates
Applications: Western Blot -
Epithelial-derived factors induce muscularis mucosa of human induced pluripotent stem cell-derived gastric organoids
Authors: K Uehara, M Koyanagi-A, T Koide, T Itoh, T Aoi
Stem Cell Reports, 2022-03-03;0(0):.
Species: Mouse
Sample Types: Tissue Homogenates
Applications: Western Blot -
Cathepsin B and D deficiency in the mouse pancreas induces impaired autophagy and chronic pancreatitis
Authors: H Iwama, S Mehanna, M Imasaka, S Hashidume, H Nishiura, KI Yamamura, C Suzuki, Y Uchiyama, E Hatano, M Ohmuraya
Scientific Reports, 2021-03-23;11(1):6596.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Neurotoxic microglia promote TDP-43 proteinopathy in progranulin deficiency
Authors: Zhang J, Velmeshev D, Hashimoto K et al.
Nature
-
LRRK2 mediates tubulation and vesicle sorting from lysosomes
Authors: L Bonet-Ponc, A Beilina, CD Williamson, E Lindberg, JH Kluss, S Saez-Atien, N Landeck, R Kumaran, A Mamais, CKE Bleck, Y Li, MR Cookson
Sci Adv, 2020-11-11;6(46):.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Network analysis of the progranulin-deficient mouse brain proteome reveals pathogenic mechanisms shared in human frontotemporal dementia caused by GRN mutations
Authors: M Huang, E Modeste, E Dammer, P Merino, G Taylor, DM Duong, Q Deng, CJ Holler, M Gearing, D Dickson, NT Seyfried, T Kukar
Acta Neuropathol Commun, 2020-10-07;8(1):163.
Species: Mouse
Sample Types: Protein
Applications: Western Blot -
Spatiotemporal proteomics uncovers cathepsin-dependent macrophage cell death during Salmonella infection
Authors: J Selkrig, N Li, A Hausmann, MSJ Mangan, M Zietek, A Mateus, J Bobonis, A Sueki, H Imamura, B El Debs, G Sigismondo, BI Florea, HS Overkleeft, N Kopitar-Je, B Turk, P Beltrao, MM Savitski, E Latz, WD Hardt, J Krijgsveld, A Typas
Nat Microbiol, 2020-06-08;0(0):.
Species: Mouse
Sample Types: Cell Lysates
Applications: Western Blot -
Neuronal Soma-Derived Degradative Lysosomes Are Continuously Delivered to Distal Axons to Maintain Local Degradation Capacity
Authors: T Farfel-Bec, JC Roney, XT Cheng, S Li, SR Cuddy, ZH Sheng
Cell Rep, 2019-07-02;28(1):51-64.e4.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
Cysteine-type cathepsins promote the effector phase of acute cutaneous delayed-type hypersensitivity reactions
Authors: J Schwenck, A Maurer, B Fehrenbach, R Mehling, P Knopf, N Mucha, D Haupt, K Fuchs, CM Griessinge, D Bukala, J Holstein, M Schaller, IG Menendez, K Ghoreschi, L Quintanill, M Gütschow, S Laufer, T Reinheckel, M Röcken, H Kalbacher, BJ Pichler, M Kneilling
Theranostics, 2019-05-31;9(13):3903-3917.
Species: Mouse
Sample Types: Tissue Lysates, Whole Cells
Applications: Flow Cytometry, Western Blot -
Early lysosomal maturation deficits in microglia triggers enhanced lysosomal activity in other brain cells of progranulin knockout mice
Authors: JK Götzl, AV Colombo, K Fellerer, A Reifschnei, G Werner, S Tahirovic, C Haass, A Capell
Mol Neurodegener, 2018-09-04;13(1):48.
Species: Mouse
Sample Types: Cell Lysates
Applications: Western Blot -
Characterization of LAMP1-labeled nondegradative lysosomal and endocytic compartments in neurons
Authors: XT Cheng, YX Xie, B Zhou, N Huang, T Farfel-Bec, ZH Sheng
J. Cell Biol., 2018-04-25;0(0):.
Species: Rat
Sample Types: Cell Culture Supernates
Applications: Western Blot -
Disruption of Small GTPase Rab7 Exacerbates the Severity of Acute Pancreatitis in Experimental Mouse Models
Authors: K Takahashi, H Mashima, K Miura, D Maeda, A Goto, T Goto, GH Sun-Wada, Y Wada, H Ohnishi
Sci Rep, 2017-06-06;7(1):2817.
Species: Mouse
Sample Types: Tissue Homogenates, Whole Tissue
Applications: IHC, Western Blot -
Deficiency for the cysteine protease cathepsin L impairs Myc-induced tumorigenesis in a mouse model of pancreatic neuroendocrine cancer.
Authors: Brindle N, Joyce J, Rostker F, Lawlor E, Swigart-Brown L, Evan G, Hanahan D, Shchors K
PLoS ONE, 2015-04-30;10(4):e0120348.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Lysosomal protein turnover contributes to the acquisition of TGFbeta-1 induced invasive properties of mammary cancer cells.
Authors: Kern U, Wischnewski V, Biniossek M, Schilling O, Reinheckel T
Mol Cancer, 2015-02-15;14(0):39.
Species: Mouse
Sample Types: Cell Lysates
Applications: Western Blot -
Eucommia ulmoides Oliver extract, aucubin, and geniposide enhance lysosomal activity to regulate ER stress and hepatic lipid accumulation.
Authors: Lee, Hwa-Youn, Lee, Geum-Hwa, Lee, Mi-Rin, Kim, Hye-Kyun, Kim, Nan-youn, Kim, Seung-Hy, Lee, Yong-Chu, Kim, Hyung-Ry, Chae, Han-Jung
PLoS ONE, 2013-12-11;8(12):e81349.
Species: Rat
Sample Types: Whole Cells
Applications: ICC -
Inflammasome activation by altered proteostasis.
Authors: Shin, Jin Na, Fattah, Elmoataz, Bhattacharya, Abhisek, Ko, Soyoung, Eissa, N Tony
J Biol Chem, 2013-10-31;288(50):35886-95.
Species: Mouse
Sample Types: Cell Lysates
Applications: Western Blot -
Macrophages and cathepsin proteases blunt chemotherapeutic response in breast cancer.
Authors: Shree T, Olson OC, Elie BT
Genes Dev., 2011-12-01;25(23):2465-79.
Species: Mouse
Sample Types: Tissue Homogenates
Applications: Western Blot -
Endocytosis provides a major alternative pathway for lysosomal biogenesis in kidney proximal tubular cells.
Authors: Nielsen R, Courtoy PJ, Jacobsen C, Dom G, Lima WR, Jadot M, Willnow TE, Devuyst O, Christensen EI
Proc. Natl. Acad. Sci. U.S.A., 2007-03-16;104(13):5407-12.
Species: Mouse
Sample Types: Serum
Applications: Western Blot -
Abl and Arg mediate cysteine cathepsin secretion to facilitate melanoma invasion and metastasis
Authors: Rakshamani Tripathi, Leann S. Fiore, Dana L. Richards, Yuchen Yang, Jinpeng Liu, Chi Wang et al.
Science Signaling
-
Lipophagy-derived fatty acids undergo extracellular efflux via lysosomal exocytosis
Authors: Cui W, Sathyanarayan A, Lopresti M et al.
Autophagy
-
Liquid-liquid phase separation facilitates the biogenesis of secretory storage granules
Authors: Parchure A, Tian M, Stalder D et al.
The Journal of cell biology
-
Neurotoxic microglia promote TDP-43 proteinopathy in progranulin deficiency
Authors: Zhang J, Velmeshev D, Hashimoto K et al.
Nature
-
Intracellular fate of carbon nanotubes inside murine macrophages: pH-dependent detachment of iron catalyst nanoparticles
Authors: Cyrill Bussy, Erwan Paineau, Julien Cambedouzou, Nathalie Brun, Claudie Mory, Barbara Fayard et al.
Particle and Fibre Toxicology
-
Translation Inhibitors Activate Autophagy Master Regulators TFEB and TFE3
Authors: TT Dang, SH Back
International Journal of Molecular Sciences, 2021-11-08;22(21):.
-
Loss of TMEM 106B potentiates lysosomal and FTLD ‐like pathology in progranulin‐deficient mice
Authors: Georg Werner, Markus Damme, Martin Schludi, Johannes Gnörich, Karin Wind, Katrin Fellerer et al.
EMBO reports
-
Increased invasiveness of MMP-9-deficient tumors in two mouse models of neuroendocrine tumorigenesis
Authors: K Shchors, H Nozawa, J Xu, F Rostker, L Swigart-Brown, G Evan et al.
Oncogene
-
Stat3 mediated alterations in lysosomal membrane protein composition
Authors: B Lloyd-Lewi, CC Krueger, TJ Sargeant, ME D'Angelo, MJ Deery, R Feret, JA Howard, KS Lilley, CJ Watson
J. Biol. Chem., 2018-01-17;0(0):.
-
Lipid‐induced lysosomal damage after demyelination corrupts microglia protective function in lysosomal storage disorders
Authors: Enrique Gabandé‐Rodríguez, Azucena Pérez‐Cañamás, Beatriz Soto‐Huelin, Daniel N Mitroi, Sara Sánchez‐Redondo, Elena Martínez‐Sáez et al.
The EMBO Journal
-
Mice Hypomorphic for Keap1, a Negative Regulator of the Nrf2 Antioxidant Response, Show Age-Dependent Diffuse Goiter with Elevated Thyrotropin Levels
Authors: Panos G. Ziros, Cédric O. Renaud, Dionysios V. Chartoumpekis, Massimo Bongiovanni, Ioannis G. Habeos, Xiao-Hui Liao et al.
Thyroid
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Mouse Cathepsin B Antibody
There are currently no reviews for this product. Be the first to review Mouse Cathepsin B Antibody and earn rewards!
Have you used Mouse Cathepsin B Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image

