Human TIM-3 PE-conjugated Antibody
Human TIM-3 PE-conjugated Antibody Summary
Ser22-Arg200
Accession # Q8TDQ0.2
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
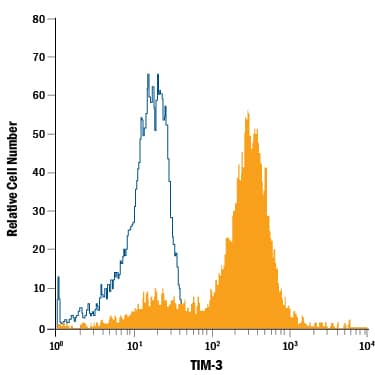
Detection of TIM‑3 in Human Blood Monocytes by Flow Cytometry. Human peripheral blood monocytes were stained with Rat Anti-Human TIM-3 PE-conjugated Monoclonal Antibody (Catalog # FAB2365P, filled histogram) or isotype control antibody (Catalog # IC006P, open histogram). View our protocol for Staining Membrane-associated Proteins.
 View Larger
View Larger
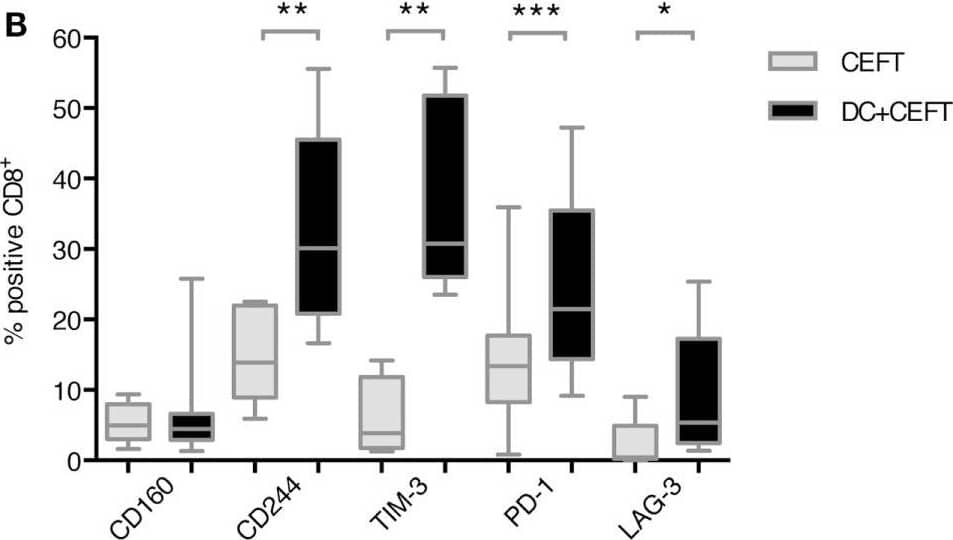
Detection of Human TIM-3 by Flow Cytometry Upregulation of immune checkpoint ligands on T cells after dendritic cell stimulation. T cells of 7–14 healthy donor were cocultured with autologous TLR-3-DCs pulsed with CMV, EBV, influenza, tetanus (CEFT) peptide pool or with CEFT peptide pool alone. Expression of various inhibitory checkpoint molecules was analyzed by flow cytometry. The percentage of positive cells is presented as box-and-whisker plots for CD4+(A) and for CD8+(B) T cells. *p < 0.05; **p < 0.01. Image collected and cropped by CiteAb from the following publication (https://journal.frontiersin.org/article/10.3389/fimmu.2018.00385/full), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
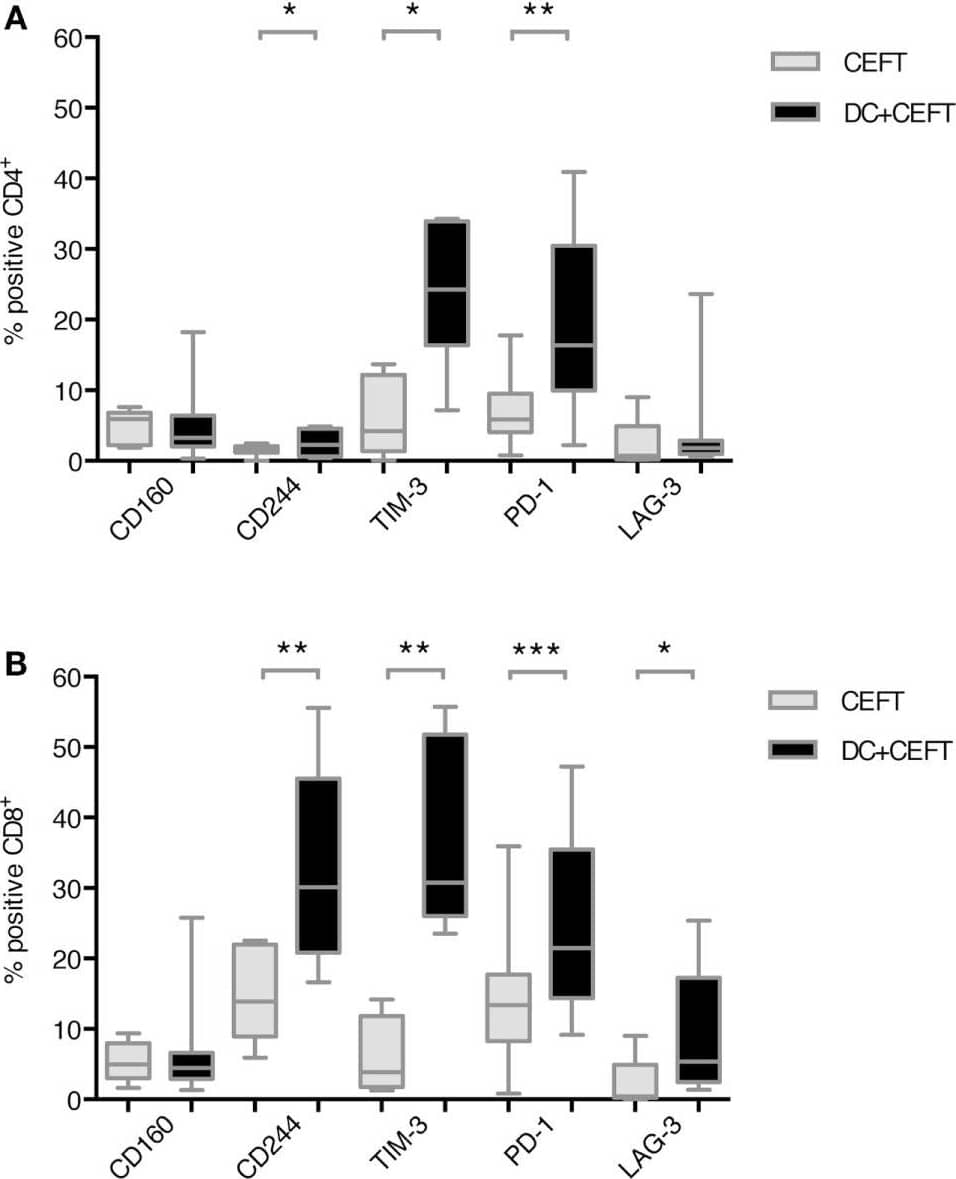
Detection of Human TIM-3 by Flow Cytometry Upregulation of immune checkpoint ligands on T cells after dendritic cell stimulation. T cells of 7–14 healthy donor were cocultured with autologous TLR-3-DCs pulsed with CMV, EBV, influenza, tetanus (CEFT) peptide pool or with CEFT peptide pool alone. Expression of various inhibitory checkpoint molecules was analyzed by flow cytometry. The percentage of positive cells is presented as box-and-whisker plots for CD4+(A) and for CD8+(B) T cells. *p < 0.05; **p < 0.01. Image collected and cropped by CiteAb from the following publication (https://journal.frontiersin.org/article/10.3389/fimmu.2018.00385/full), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
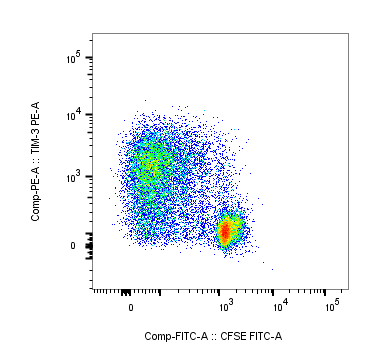
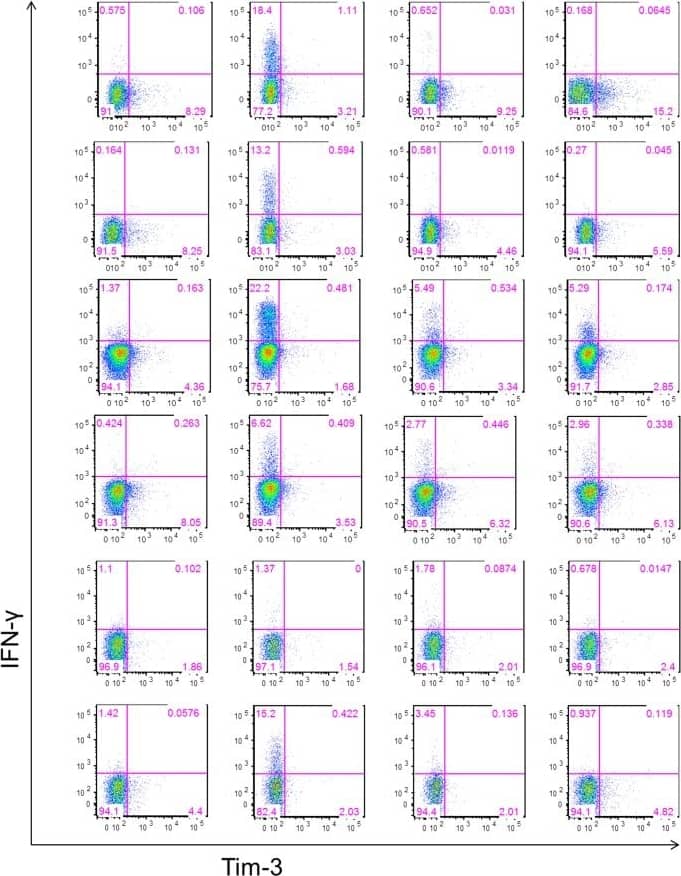
Detection of Human TIM-3 by Flow Cytometry Gating strategy for phenotyping IFN-gamma secreting T cells in response to stimulation.The frequencies of Tim-3+ and Tim-3− IFN-gamma secreting CD4+ and CD8+ T cells were measured in PBMC collected at day 30 post-index from 6 HLA-A02 WNV-infected donors and incubated with or without anti-CD3/anti-CD28 mAbs, WNV peptide pool, and SVG9 tetramer. Tim-3− and Tim-3+ CD4+ and CD8+ T cells were analyzed for IFN-gamma secretion. The gates were set using fluorescence minus one controls. Dot-plots for CD8+ T cells from all 6 WNV+ subjects in different stimulation conditions are shown. Image collected and cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0092134), licensed under a CC-BY license. Not internally tested by R&D Systems.
Preparation and Storage
- 12 months from date of receipt, 2 to 8 °C as supplied.
Background: TIM-3
TIM-3 (T cell immunoglobulin and mucin domain-3), also known as HAVCR2, is a 60 kDa member of the TIM family of immune regulating molecules. TIMs are type I transmembrane glycoproteins with one Ig-like V-type domain and a Ser/Thr-rich mucin stalk region (1, 2). Mature human TIM-3 consists of a 181 amino acid (aa) extracellular domain (ECD), a 21 aa transmembrane segment, and a 78 aa cytoplasmic tail (3). An alternatively spliced isoform is truncated within the mucin-like stalk. Within the ECD, human TIM-3 shares 58% aa sequence identity with mouse and rat TIM-3. TIM-3 is up‑regulated on several populations of activated myeloid cells (macrophage, monocyte, dendritic cell, microglia, mast cell) and T cells (Th1, CD8+, NK, Treg) (3-10). Its binding to Galectin-9 induces a range of immunosuppressive functions which enhance immune tolerance and inhibit anti-tumor immunity (11). TIM-3 ligation attenuates CD8+ and Th1 cell responses (11-13) and promotes the activity of Treg and myeloid derived suppressor cells (8, 11, 13, 14). In addition, dendritic cell-expressed TIM-3 dampens inflammation by enabling the phagocytosis of apoptotic cells and the cross-presentation of apoptotic cell antigens (4). It also binds the alarmin HMGB1, thereby preventing the activation of TLRs in response to released tumor cell DNA (7). TIM-3 interactions with Galectin-9 can alternatively trigger immune stimulatory effects, such as the coactivation of NK cell cytotoxicity (10).
- Sakuishi, K. et al. (2011) Trends Immunol. 32:345.
- Anderson, A.C. (2012) Curr. Opin. Immunol. 24:213.
- Monney, L. et al. (2002) Nature 415:536.
- Nakayama, M. et al. (2009) Blood 113:3821.
- Anderson, A.C. et al. (2007) Science 318:1141.
- Wiener, Z. et al. (2007) J. Invest. Dermatol. 127:906.
- Chiba, S. et al. (2012) Nat. Immunol. 13:832.
- Sanchez-Fueyo, A. et al. (2003) Nat. Immunol. 4:1093.
- Ndhlovu, L.C. et al. (2012) Blood 119:3734.
- Gleason, M.K. et al. (2012) Blood 119:3064.
- Zhu, C. et al. (2005) Nat. Immunol. 6:1245.
- Sakhdari, A. et al. (2012) PLoS ONE 7:e40146.
- Sabatos, C.A. et al. (2003) Nat. Immunol. 4:1102.
- Dardalhon, V. et al. (2010) J. Immunol. 185:1383.
Product Datasheets
Citations for Human TIM-3 PE-conjugated Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
37
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Evaluation of the Elements of Short Hairpin RNAs in Developing shRNA-Containing CAR T Cells
Authors: Urak, R;Gittins, B;Soemardy, C;Grepo, N;Goldberg, L;Maker, M;Shevchenko, G;Davis, A;Li, S;Scott, T;Morris, KV;Forman, SJ;Wang, X;
Cancers
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Phosphatidylserine binding directly regulates TIM-3 function
Authors: Courtney M. Smith, Alice Li, Nithya Krishnamurthy, Mark A. Lemmon
Biochemical Journal
-
Association of TIM-3 expression with glucose metabolism in Jurkat T cells
Authors: MJ Lee, SJ Yun, B Lee, E Jeong, G Yoon, K Kim, S Park
BMC Immunol., 2020-08-20;21(1):48.
Species: Human
Sample Types: Transfected Whole Cells, Whole Cells
Applications: Flow Cytometry -
Different Expression Pattern of TIM-3 and Galectin-9 Molecules by Peripheral and Peritoneal Lymphocytes in Women with and without Endometriosis
Authors: M Meggyes, L Szereday, N Bohonyi, M Koppan, S Szegedi, A Marics-Kut, M Marton, A Totsimon, B Polgar
Int J Mol Sci, 2020-03-28;21(7):.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
T cell receptor gene therapy targeting WT1 prevents acute myeloid leukemia relapse post-transplant
Authors: AG Chapuis, DN Egan, M Bar, TM Schmitt, MS McAfee, KG Paulson, V Voillet, R Gottardo, GB Ragnarsson, M Bleakley, CC Yeung, P Muhlhauser, HN Nguyen, LA Kropp, L Castelli, F Wagener, D Hunter, M Lindberg, K Cohen, A Seese, MJ McElrath, N Duerkopp, TA Gooley, PD Greenberg
Nat. Med., 2019-06-24;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Co-expression of CD39 and CD103 identifies tumor-reactive CD8 T cells in human solid tumors
Authors: T Duhen, R Duhen, R Montler, J Moses, T Moudgil, NF de Miranda, CP Goodall, TC Blair, BA Fox, JE McDermott, SC Chang, G Grunkemeie, R Leidner, RB Bell, AD Weinberg
Nat Commun, 2018-07-13;9(1):2724.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Phase I trial of plerixafor combined with decitabine in newly diagnosed older patients with acute myeloid leukemia
Authors: GJ Roboz, EK Ritchie, Y Dault, L Lam, DC Marshall, NM Cruz, HC Hsu, DC Hassane, PJ Christos, C Ippoliti, JM Scandura, ML Guzman
Haematologica, 2018-05-03;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Targeting LAG-3 and PD-1 to Enhance T Cell Activation by Antigen-Presenting Cells
Authors: Felix S. Lichtenegger, Maurine Rothe, Frauke M. Schnorfeil, Katrin Deiser, Christina Krupka, Christian Augsberger et al.
Frontiers in Immunology
-
Frontline Science: Tim-3-mediated dysfunctional engulfment of apoptotic cells in SLE
Authors: D Zhao, M Guo, B Liu, Q Lin, T Xie, Q Zhang, X Jia, Q Shu, X Liang, L Gao, C Ma
J. Leukoc. Biol., 2017-07-28;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
TCR-ligand dissociation rate is a robust and stable biomarker of CD8+ T cell potency
Authors: M Allard, B Couturaud, L Carretero-, MN Duong, J Schmidt, GC Monnot, P Romero, DE Speiser, M Hebeisen, N Rufer
JCI Insight, 2017-07-20;2(14):.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Synergistic effect of IL-12 and IL-18 induces TIM3 regulation of ?? T cell function and decreases the risk of clinical malaria in children living in Papua New Guinea
Authors: L Schofield, LJ Ioannidis, S Karl, LJ Robinson, QY Tan, DP Poole, I Betuela, DL Hill, PM Siba, DS Hansen, I Mueller, EM Eriksson
BMC Med, 2017-06-15;15(1):114.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
PD-1 Blockade Promotes Emerging Checkpoint Inhibitors in Enhancing T Cell Responses to Allogeneic Dendritic Cells
Authors: C Stecher, C Battin, J Leitner, M Zettl, K Grabmeier-, C Höller, GJ Zlabinger, P Steinberge
Front Immunol, 2017-05-22;8(0):572.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
BCG vaccination induces HIV target cell activation in HIV-exposed infants in a randomized trial
Authors: MA Gasper, AC Hesseling, I Mohar, L Myer, T Azenkot, JS Passmore, W Hanekom, MF Cotton, IN Crispe, DL Sodora, HB Jaspan
JCI Insight, 2017-04-06;2(7):e91963.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Tim-3 is a Marker of Plasmacytoid Dendritic Cell Dysfunction during HIV Infection and Is Associated with the Recruitment of IRF7 and p85 into Lysosomes and with the Submembrane Displacement of TLR9
Authors: JA Schwartz, KL Clayton, S Mujib, H Zhang, AK Rahman, J Liu, FY Yue, E Benko, C Kovacs, MA Ostrowski
J. Immunol, 2017-03-06;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Dendritic cell vaccination in combination with docetaxel for patients with metastatic castration-resistant prostate cancer: A randomized phase II study.
Authors: Kongsted P, Borch T, Ellebaek E, Iversen T, Andersen R, Met O, Hansen M, Lindberg H, Sengelov L, Svane I
Cytotherapy, 2017-02-15;19(4):500-513.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition
J Clin Invest, 2016-07-25;126(8):3130-44.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
CD62L+ NKT cells have prolonged persistence and antitumor activity in vivo
J Clin Invest, 2016-05-16;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
CD3(bright)CD56(+) T cells associate with pegylated interferon-alpha treatment nonresponse in chronic hepatitis B patients
Sci Rep, 2016-05-13;6(0):25567.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Polyfunctional Melan-A-specific tumor-reactive CD8(+) T cells elicited by dacarbazine treatment before peptide-vaccination depends on AKT activation sustained by ICOS
Authors: Ornella Franzese, Belinda Palermo, Cosmo Di Donna, Isabella Sperduti, Virginia Ferraresi, Helena Stabile et al.
OncoImmunology
-
GPR56 identifies primary human acute myeloid leukemia cells with high repopulating potential in vivo
Authors: Caroline Pabst, Anne Bergeron, Vincent-Philippe Lavallée, Jonathan Yeh, Patrick Gendron, Gudmundur L. Norddahl et al.
Blood
-
Expression of the galectin-9-Tim-3 pathway in glioma tissues is associated with the clinical manifestations of glioma
Authors: ZENGJIN LIU, HUAMIN HAN, XIN HE, SHOUWEI LI, CHENXING WU, CHUNJIANG YU et al.
Oncology Letters
-
Multidimensional Clusters of CD4+ T Cell Dysfunction Are Primarily Associated with the CD4/CD8 Ratio in Chronic HIV Infection.
Authors: Frederiksen J, Buggert M, Noyan K, Nowak P, Sonnerborg A, Lund O, Karlsson A
PLoS ONE, 2015-09-24;10(9):e0137635.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
CD127 expression, exhaustion status and antigen specific proliferation predict sustained virologic response to IFN in HCV/HIV co-infected individuals.
Authors: Kared H, Saeed S, Klein M, Shoukry N
PLoS ONE, 2014-07-09;9(7):e101441.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Peripheral blood TIM-3 positive NK and CD8+ T cells throughout pregnancy: TIM-3/galectin-9 interaction and its possible role during pregnancy.
Authors: Meggyes, Matyas, Miko, Eva, Polgar, Beata, Bogar, Barbara, Farkas, Balint, Illes, Zsolt, Szereday, Laszlo
PLoS ONE, 2014-03-20;9(3):e92371.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Increased frequency of Tim-3 expressing T cells is associated with symptomatic West Nile virus infection.
Authors: Lanteri M, Diamond M, Law J, Chew G, Wu S, Inglis H, Wong D, Busch M, Norris P, Ndhlovu L
PLoS ONE, 2014-03-18;9(3):e92134.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Fusion of ubiquitin to HIV gag impairs human monocyte-derived dendritic cell maturation and reduces ability to induce gag T cell responses.
Authors: Herath S, Benlahrech A, Papagatsias T, Athanasopoulos T, Bouzeboudjen Z, Hervouet C, Klavinskis L, Meiser A, Kelleher P, Dickson G, Patterson S
PLoS ONE, 2014-02-05;9(2):e88327.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
T cell Ig and mucin domain-containing protein 3 is recruited to the immune synapse, disrupts stable synapse formation, and associates with receptor phosphatases.
Authors: Clayton K, Haaland M, Douglas-Vail M, Mujib S, Chew G, Ndhlovu L, Ostrowski M
J Immunol, 2013-12-13;192(2):782-91.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Isolation and characterization of canine natural killer cells
Authors: Helen T. Michael, Daisuke Ito, Valarie McCullar, Bin Zhang, Jeffrey S. Miller, Jaime F. Modiano
Veterinary Immunology and Immunopathology
Species: Canine
Sample Types: Whole Cells
Applications: Flow Cytometry -
The immunomodulatory effects of bevacizumab on systemic immunity in patients with metastatic melanoma.
Authors: Mansfield A, Nevala W, Lieser E, Leontovich A, Markovic S
Oncoimmunology, 2013-05-01;2(5):e24436.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Upregulation of the tim-3/galectin-9 pathway of T cell exhaustion in chronic hepatitis B virus infection.
Authors: Nebbia G, Peppa D, Schurich A, Khanna P, Singh H, Cheng Y, Rosenberg W, Dusheiko G, Gilson R, Chinaleong J, Kennedy P, Maini M
PLoS ONE, 2012-10-24;7(10):e47648.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Age-related expansion of Tim-3 expressing T cells in vertically HIV-1 infected children.
Authors: Tandon, Ravi, Giret, Maria T, Sengupta, Devi, York, Vanessa, Wiznia, Andrew A, Rosenberg, Michael, Kallas, Esper G, Ndhlovu, Lishomwa, Nixon, Douglas
PLoS ONE, 2012-09-24;7(9):e45733.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Tim-3 Negatively Regulates Cytotoxicity in Exhausted CD8(+) T Cells in HIV Infection.
Authors: Sakhdari A, Mujib S, Vali B
PLoS ONE, 2012-07-05;7(7):e40146.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Antigen-independent induction of Tim-3 expression on human T cells by the common gamma-chain cytokines IL-2, IL-7, IL-15, and IL-21 is associated with proliferation and is dependent on the phosphoinositide 3-kinase pathway.
Authors: Mujib S, Jones RB, Lo C, Aidarus N, Clayton K, Sakhdari A, Benko E, Kovacs C, Ostrowski MA
J. Immunol., 2012-03-14;188(8):3745-56.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Extended co-expression of inhibitory receptors by human CD8 T-cells depending on differentiation, antigen-specificity and anatomical localization.
Authors: Baitsch L, Legat A, Barba L, Fuertes Marraco SA, Rivals JP, Baumgaertner P, Christiansen-Jucht C, Bouzourene H, Rimoldi D, Pircher H, Rufer N, Matter M, Michielin O, Speiser DE
PLoS ONE, 2012-02-08;7(2):e30852.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Elevated frequencies of highly activated CD4+ T cells in HIV+ patients developing immune reconstitution inflammatory syndrome.
Authors: Antonelli LR, Mahnke Y, Hodge JN, Porter BO, Barber DL, DerSimonian R, Greenwald JH, Roby G, Mican J, Sher A, Roederer M, Sereti I
Blood, 2010-07-26;116(19):3818-27.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Altered expression of T cell immunoglobulin-mucin (TIM) molecules in bronchoalveolar lavage CD4+ T cells in sarcoidosis.
Authors: Idali F, Wahlstrom J, Dahlberg B, Khademi M, Olsson T, Eklund A, Grunewald J
Respir Res, 2009-05-29;10(0):42.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
New insights into the phenotype of human dendritic cell populations.
Authors: Clark GJ et al.
Clin Transl Immunology
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Human TIM-3 PE-conjugated Antibody
Average Rating: 5 (Based on 1 Review)
Have you used Human TIM-3 PE-conjugated Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by: