Human/Mouse/Rat Neurofascin Antibody Summary
Ile25-Ala1031
Accession # NP_446361
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
Detection of Human, Mouse, and Rat Neurofascin by Western Blot. Western blot shows lysates of rat brain tissue, mouse brain tissue, and human brain (cerebellum). PVDF Membrane was probed with 0.1 µg/mL of Chicken Anti-Human/Mouse/Rat Neurofascin Antigen Affinity-purified Polyclonal Antibody (Catalog # AF3235) followed by HRP-conjugated Anti-Chicken IgY Secondary Antibody. Specific bands were detected for Neurofascin at approximately 140 and186 kDa (as indicated). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 1.
 View Larger
View Larger
Neurofascin in Rat Brain. Neurofascin was detected in perfusion fixed frozen sections of rat brain (dorsal root ganglion) using Chicken Anti-Human/Mouse/Rat Neurofascin Antigen Affinity-purified Polyclonal Antibody (Catalog # AF3235) at 1.7 µg/mL overnight at 4 °C. Tissue was stained using the NorthernLights™ 557-conjugated Anti-Chicken IgY Secondary Antibody (yellow; Catalog # NL016) and counterstained with DAPI (blue). Specific staining was localized to neuronal cell bodies and Schwann cells (perinodal regions). View our protocol for Fluorescent IHC Staining of Frozen Tissue Sections.
 View Larger
View Larger
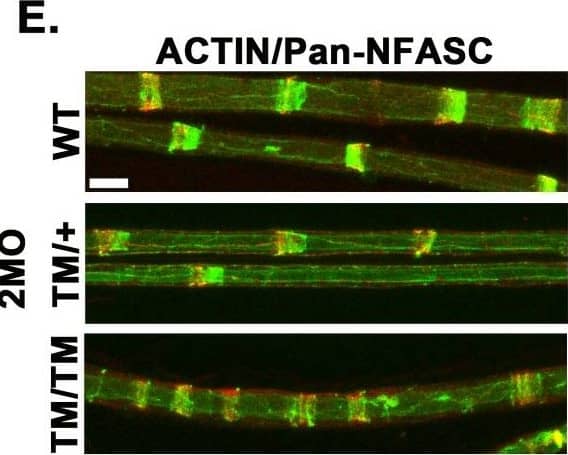
Detection of Mouse Neurofascin by Immunohistochemistry P0T124M mutation alters SLI morphology, length,&distribution. (D) Quantification shows an increased %age of SLIs in nerves from mice harboring the P0T124M mutation (MpzT124M) at 2, 6, 12,&18 months of age. One-way ANOVA [2 month old: F (2, 10) = 29.86, p<0.0001; 6 month old: F (2, 9) = 41.60, p<0.0001, 12 month old: F (2, 6) = 34.35, p = 0.0005; 18 month old: F (2, 8) = 21.71, p = 0.0006]. Representative confocal pictures of sciatic teased fibers stained with FITC-phalloidin (ACTIN, green) (E, I,&M) or anti-MAG antibodies (Q) (green)&anti-pan-NFASC antibodies (red) at 2 (E), 6 (I),&12 (M&Q) months of age. SLI morphology is disrupted in MpzT124M mice. Scale bar: 10 μm. Measurements of SLI length at 2 (F), 6 (J),&12 (N&R) months of age. Nested one-way ANOVA [2 month old: F (2,9) = 32.16, p <0.0001; 6 month old: F (2,6) = 11.18, p = 0.0095 12 month old: F (2,9) = 6.447, p = 0.0183]. Measurements of the distance between adjacent SLI at 2 (G), 6 (K),&12 (O&S) months of age. Nested one-way ANOVA [2 month old: F (2,9) = 24.90, p = 0.0002; 6 month old: F (2,6) = 9.382, p = 0.0142; 12 month old: F (2,9) = 13.04, p = 0.0022]. Quantifications of SLI number per 100 μm at 2 (H), 6 (L),&12 (P&T) months of age. Nested one-way ANOVA [2 month old: F (2,9) = 20.29, p = 0.0005; 6 month old: F (2,6) = 22.81, p = 0.016; 12 month old: F (2,9) = 28.25, p = 0.0001]. At least 207 SLI per genotype quantified at each time point. n (animals) ≥ 3 per genotype. *p < 0.05, **p < 0.01, ***p < 0.001 by multiple-comparisons Tukey’s post hoc tests after Nested one-way ANOVA (D, F, G, H, J, K, L, R, S,&T) or by two-tailed Student’s t test (N, O,&P). Graphs indicate means ± SEMs. Image collected & cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36350884), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
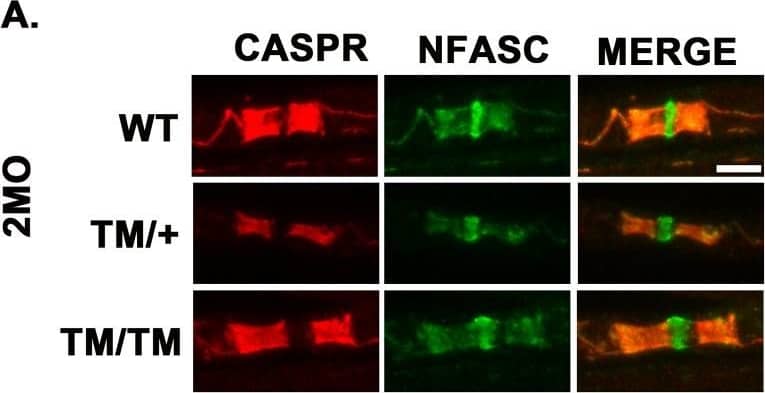
Detection of Mouse Neurofascin by Immunohistochemistry P0T124M mutation alters nodes and paranodes.Representative confocal pictures of sciatic nerve teased fibers stained with antibodies against the paranodal marker CASPR (red) and the paranodal and nodal marker pan-Neurofascin (NFASC; green) at 2 (A) and 12 (E) months of age. Scale bars: 5 μm. CASPR length quantifications at 2 (B) and 12 (F) months of age. Nested one-way ANOVA [2 month old: F (2,9) = 7.009, p = 0.0146; 12 month old: F (2,7) = 35.47, p = 0.0002]. Relative frequency distributions of paranodal (CASPR) length at 2 (C) and 12 (G) months of age. Nodal length quantifications at 2 (D) and 12 (H) months of age. Nested one-way ANOVA [2 month old: F (2,9) = 1.122, p = 0.3671, 12 month old: F (2,7) = 8.012, p = 0.0155]. At least 200 paranodes and 100 nodes per genotype were quantified at each time point. n (animals) ≥ 3 per genotype. (I) Electron micrographs of ultrathin longitudinal WT and MpzT124M/T124M sciatic nerve section at 12 months of age. In (I) electron micrographs represent nodes of WT and MpzT124M/T124M sciatic nerves. (J) The quantification of nodal length shows significant widening of the node in MpzT124M/T124M. Magnifications of (I) show disorganized paranonal loops in MpzT124M/T124M but well organized in WT. n (animals) ≥ 3 per genotype, 7 to 11 nodes were counted per animals, for a total of 27 and 28 counted by genotype. Scale bare: top panel 1 μm, middle panel 500 nm, bottom panel 200 nm. ***p < 0.001 by multiple-comparisons Tukey’s post hoc tests after Nested one-way ANOVA (B, F, D, and H) or Nested two-tailed Student’s t test (J). Graphs indicate means ± SEMs. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36350884), licensed under a CC-BY license. Not internally tested by R&D Systems.
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: Neurofascin
Neurofascin 155 (NF155) is a type I transmembrane glycoprotein that belongs to the L1CAM family of cell adhesion proteins (1, 2). The rat NF155 cDNA encodes a 1240 amino acid (aa) precursor that contains a 24 aa signal sequence, a 1086 aa extracellular domain (ECD), a 21 aa transmembrane segment, and a 109 aa cytoplasmic domain. The ECD consists of six Ig-like domains and four fibronectin type III repeats, the second of which has an RGD motif. A splice variant of Neurofascin, NF186, lacks the RGD-containing fibronectin type III domain but instead has a mucin-like domain and an additional non-RGD fibronectin type III domain (3). Within shared regions of the ECD, rat NF155 shares 45% and 39% aa sequence identity with rat Nr-CAM and L1CAM, respectively, and 98% aa sequence identity with human and mouse NF155. NF155 is transiently expressed by oligodendrocytes at the onset of axon myelination, whereas NF186 is neuronally expressed in nodes of Ranvier (4‑6). Clustering of NF155 in paranodal oligodengroglia lipid raft domains is stabilized by dimerization of its cytoplasmic domains and association with intracellular ankyrin (6‑9). NF155 interacts with axonal contactin and plays a role in node of Ranvier formation and the establishment of saltatory conduction (5, 9‑12). The ECD of NF155 is cleaved from oligodengroglia membranes by metalloproteases, a process which is required for NF155 transport from the glial cell body to the axoglial junction (13). In addition to distinct expression patterns, Neurofascin isoforms have different functional properties. NF155 promotes neuronal adhesion and neurite outgrowth, whereas NF186 inhibits neuronal adhesion (4, 7, 13).
- Sherman, D.L. and P.J. Brophy (2005) Nat. Rev. Neurosci. 6:683.
- Coman, I. et al. (2005) J. Neurol. Sci. 233:67.
- Volkmer, H. et al. (1992) J. Cell Biol. 118:149.
- Koticha, D. et al. (2005) Mol. Cell. Neurosci. 30:137.
- Collinson, J.M. et al. (1998) Glia 23:11.
- Schafer, D.P. et al. (2004) J. Neurosci. 24:3176.
- Maier, O. et al. (2005) Mol. Cell. Neurosci. 28:390.
- Zhang, X. et al. (1998) J. Biol. Chem. 273:30785.
- Sherman, D.L. et al. (2005) Neuron 48:737.
- Gollan, L. et al. (2003) J. Cell Biol. 163:1213.
- Charles, P. et al. (2002) Curr. Biol. 12:217.
- Volkmer, H. et al. (1996) J. Cell Biol. 135:1059.
- Maier, O. et al. (2006) Exp. Cell Res. 312:500.
Product Datasheets
Citations for Human/Mouse/Rat Neurofascin Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
36
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Reduced length of nodes of Ranvier and altered proteoglycan immunoreactivity in prefrontal white matter in major depressive disorder and chronically stressed rats
Authors: José Javier Miguel-Hidalgo, Erik Hearn, Mohadetheh Moulana, Khunsa Saleem, Austin Clark, Maggie Holmes et al.
Sci Rep
-
Loss of TMEM106B and PGRN leads to severe lysosomal abnormalities and neurodegeneration in mice
Authors: Feng T, Mai S, Roscoe JM et al.
EMBO Rep.
-
Antibodies against nodo-paranodal proteins are not present in genetic neuropathies
Authors: Lorena Martín-Aguilar, Elba Pascual-Goñi, Cinta Lleixà, Marina Frasquet, Herminia Argente, Angel Cano-Abascal et al.
Neurology
-
The Polarity Protein Pals1 Regulates Radial Sorting of Axons
Authors: Daniel R Zollinger, Kae-Jiun Chang, Kelli Baalman, Seonhee Kim, Matthew N Rasband
Journal of Neuroscience
-
Type 2 Diabetes Leads to Axon Initial Segment Shortening in db/db Mice
Authors: Leonid M. Yermakov, Domenica E. Drouet, Ryan B. Griggs, Khalid M. Elased, Keiichiro Susuki
Frontiers in Cellular Neuroscience
-
Ankyrin-R regulates fast-spiking interneuron excitability through perineuronal nets and Kv3.1b K(+) channels
Authors: Stevens SR, Longley CM, Ogawa Y et al.
eLife
-
beta spectrin-dependent and domain specific mechanisms for Na+ channel clustering
Authors: Cheng-Hsin Liu, Ryan Seo, Tammy Szu-Yu Ho, Michael Stankewich, Peter J Mohler, Thomas J Hund et al.
eLife
-
Diabetes Mellitus Is a Possible Risk Factor for Nodo-paranodopathy With Antiparanodal Autoantibodies
Authors: Luise Appeltshauser, Julia Messinger, Katharina Starz, David Heinrich, Anna-Michelle Brunder, Helena Stengel et al.
Neurology - Neuroimmunology Neuroinflammation
-
Glial M6B stabilizes the axonal membrane at peripheral nodes of Ranvier
Authors: Marie L Bang, Anya Vainshtein, Hyun-Jeong Yang, Yael Eshed-Eisenbach, Jerome Devaux, Hauke B Werner et al.
Glia
-
Endogenously expressed Ranbp2 is not at the axon initial segment
Authors: Yuki Ogawa, Matthew N. Rasband
Journal of Cell Science
-
A hierarchy of PDZ domain scaffolding proteins clusters the Kv1 K+ channel protein complex at the axon initial segment
Authors: Zhang, W;Palfini, VL;Wu, Y;Ding, X;Melton, AJ;Gao, Y;Ogawa, Y;Rasband, MN;
Science advances
Species: Rat
Sample Types: Whole Cells
Applications: Immunocytochemistry -
Antibody-directed extracellular proximity biotinylation reveals that Contactin-1 regulates axo-axonic innervation of axon initial segments
Authors: Ogawa, Y;Lim, BC;George, S;Oses-Prieto, JA;Rasband, JM;Eshed-Eisenbach, Y;Hamdan, H;Nair, S;Boato, F;Peles, E;Burlingame, AL;Van Aelst, L;Rasband, MN;
Nature communications
Species: Rat
Sample Types: Whole Cells
Applications: ICC -
Compromised Myelin and Axonal Molecular Organization Following Adult-Onset Sulfatide Depletion
Authors: Dustin, E;Suarez-Pozos, E;Stotesberry, C;Qiu, S;Palavicini, JP;Han, X;Dupree, JL;
Biomedicines
Species: Mouse
Sample Types: Tissue Homogenates
Applications: Western Blot -
Antibody-directed extracellular proximity biotinylation reveals Contactin-1 regulates axo-axonic innervation of axon initial segments
Authors: Y Ogawa, BC Lim, S George, JA Oses-Priet, JM Rasband, Y Eshed-Eise, S Nair, F Boato, E Peles, AL Burlingame, LV Aelst, MN Rasband
bioRxiv : the preprint server for biology, 2023-03-06;0(0):.
Species: Rat
Sample Types: Whole Cells
Applications: Biotinylation, ICC -
Distinct Changes in Calpain and Calpastatin during PNS Myelination and Demyelination in Rodent Models
Authors: JA Miller, DE Drouet, LM Yermakov, MS Elbasiouny, FZ Bensabeur, M Bottomley, K Susuki
International Journal of Molecular Sciences, 2022-12-06;23(23):.
Species: Rat
Sample Types: Whole Tissue
Applications: IHC -
A new mouse model of Charcot-Marie-Tooth 2J neuropathy replicates human axonopathy and suggest alteration in axo-glia communication
Authors: G Shacklefor, LN Marziali, Y Sasaki, A Claessens, C Ferri, NI Weinstock, AM Rossor, NJ Silvestri, ER Wilson, E Hurley, GJ Kidd, S Manohar, D Ding, RJ Salvi, ML Feltri, M D'Antonio, L Wrabetz
PloS Genetics, 2022-11-09;18(11):e1010477.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Ankyrin-R regulates fast-spiking interneuron excitability through perineuronal nets and Kv3.1b K(+) channels
Authors: Stevens SR, Longley CM, Ogawa Y et al.
eLife
-
Prohibitin 1 is essential to preserve mitochondria and myelin integrity in Schwann cells
Authors: G Della-Flor, ER Wilson, LN Marziali, E Hurley, N Silvestri, B He, BW O'Malley, B Beirowski, Y Poitelon, L Wrabetz, ML Feltri
Nature Communications, 2021-06-02;12(1):3285.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
TDP-43 maximizes nerve conduction velocity by repressing a cryptic exon for paranodal junction assembly in Schwann cells
Authors: KJ Chang, I Agrawal, A Vainshtein, WY Ho, W Xin, G Tucker-Kel, K Susuki, E Peles, SC Ling, JR Chan
Elife, 2021-03-10;10(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Immunoadsorption and Plasma Exchange in Seropositive and Seronegative Immune-Mediated Neuropathies
Authors: AJ Davies, J Fehmi, M Senel, H Tumani, J Dorst, S Rinaldi
J Clin Med, 2020-06-27;9(7):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Accumulation of Neurofascin at nodes of Ranvier is regulated by a Paranodal Switch
Authors: Y Zhang, S Yuen, E Peles, JL Salzer
J. Neurosci., 2020-06-17;0(0):.
Species: Transgenic Mouse
Sample Types: Cell Lysates
Applications: Western Blot -
Impairment of cognitive flexibility in type 2 diabetic db/db mice
Authors: Leonid M. Yermakov, Ryan B. Griggs, Domenica E. Drouet, Chiho Sugimoto, Michael T. Williams, Charles V. Vorhees et al.
Behavioural Brain Research
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Glial ?II spectrin contributes to paranode formation and maintenance
Authors: K Susuki, DR Zollinger, KJ Chang, C Zhang, CY Huang, CR Tsai, MR Galiano, Y Liu, SD Benusa, LM Yermakov, RB Griggs, JL Dupree, MN Rasband
J. Neurosci., 2018-05-31;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Methylglyoxal Disrupts Paranodal Axoglial Junctions via Calpain Activation
Authors: RB Griggs, LM Yermakov, DE Drouet, DVM Nguyen, K Susuki
ASN Neuro, 2018-01-01;10(0):1759091418766.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Antibodies against peripheral nerve antigens in chronic inflammatory demyelinating polyradiculoneuropathy
Authors: L Querol, AM Siles, R Alba-Rovir, A Jáuregui, J Devaux, C Faivre-Sar, J Araque, R Rojas-Garc, J Diaz-Maner, E Cortés-Vic, G Nogales-Ga, M Navas-Madr, E Gallardo, I Illa
Sci Rep, 2017-10-31;7(1):14411.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
The paranodal cytoskeleton clusters Na(+) channels at nodes of Ranvier
Authors: V Amor, C Zhang, A Vainshtein, A Zhang, DR Zollinger, Y Eshed-Eise, PJ Brophy, MN Rasband, E Peles
Elife, 2017-01-30;6(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
A hierarchy of ankyrin-spectrin complexes clusters sodium channels at nodes of Ranvier
Authors: Tammy Szu-Yu Ho, Daniel R. Zollinger, Kae-Jiun Chang, Mingxuan Xu, Edward C. Cooper, Michael C. Stankewich et al.
Nature Neuroscience
Species: Mouse
Sample Types: Whole Cells
Applications: Immunocytochemistry -
Glial ankyrins facilitate paranodal axoglial junction assembly
Authors: Kae-Jiun Chang, Daniel R. Zollinger, Keiichiro Susuki, Diane L. Sherman, Michael A. Makara, Peter J. Brophy et al.
Nature Neuroscience
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Nodal beta spectrins are required to maintain Na+ channel clustering and axon integrity
Authors: Liu CH, Stevens SR, Teliska LH et al.
Elife
-
Neurofascin Is a Novel Component of Rod Photoreceptor Synapses in the Outer Retina
Authors: Sahar Pourhoseini, Debalina Goswami-Sewell, Elizabeth Zuniga-Sanchez
Frontiers in Neural Circuits
-
Glial ankyrins facilitate paranodal axoglial junction assembly
Authors: Kae-Jiun Chang, Daniel R. Zollinger, Keiichiro Susuki, Diane L. Sherman, Michael A. Makara, Peter J. Brophy et al.
Nature Neuroscience
-
A hierarchy of ankyrin-spectrin complexes clusters sodium channels at nodes of Ranvier
Authors: Tammy Szu-Yu Ho, Daniel R. Zollinger, Kae-Jiun Chang, Mingxuan Xu, Edward C. Cooper, Michael C. Stankewich et al.
Nature Neuroscience
-
An alpha II Spectrin-Based Cytoskeleton Protects Large-Diameter Myelinated Axons from Degeneration
Authors: Claire Yu-Mei Huang, Chuansheng Zhang, Daniel R. Zollinger, Christophe Leterrier, Matthew N. Rasband
The Journal of Neuroscience
-
NuMA1 promotes axon initial segment assembly through inhibition of endocytosis
Authors: Tomohiro Torii, Yuki Ogawa, Cheng-Hsin Liu, Tammy Szu-Yu Ho, Hamdan Hamdan, Chih-Chuan Wang et al.
J. Cell Biol
-
Impairment of cognitive flexibility in type 2 diabetic db/db mice
Authors: Leonid M. Yermakov, Ryan B. Griggs, Domenica E. Drouet, Chiho Sugimoto, Michael T. Williams, Charles V. Vorhees et al.
Behavioural Brain Research
-
m6A mRNA Methylation Is Essential for Oligodendrocyte Maturation and CNS Myelination
Authors: Huan Xu, Yulia Dzhashiashvili, Ankeeta Shah, Rejani B. Kunjamma, Yi-lan Weng, Benayahu Elbaz et al.
Neuron
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Human/Mouse/Rat Neurofascin Antibody
There are currently no reviews for this product. Be the first to review Human/Mouse/Rat Neurofascin Antibody and earn rewards!
Have you used Human/Mouse/Rat Neurofascin Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
