Human MMP-1 Antibody Summary
Phe20-Asn469
Accession # P03956
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
Detection of Human MMP‑1 by Western Blot. Western blot shows lysates of PC-3 human prostate cancer cell line. PVDF Membrane was probed with 2 µg/mL of Mouse Anti-Human MMP-1 Monoclonal Antibody (Catalog # MAB901) followed by HRP-conjugated Anti-Mouse IgG Secondary Antibody (Catalog # HAF007). A specific band was detected for MMP-1 at approximately 54 kDa (as indicated). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 1.
 View Larger
View Larger
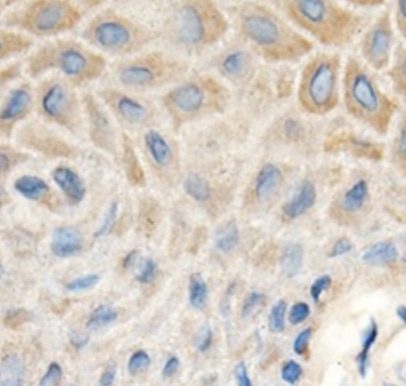
MMP‑1 in Human Ovarian Cancer Tissue. MMP‑1 was detected in immersion fixed paraffin-embedded sections of human ovarian cancer tissue using 25 µg/mL Mouse Anti-Human MMP‑1 Monoclonal Antibody (Catalog # MAB901) overnight at 4 °C. Tissue was stained with the Anti-Mouse HRP-AEC Cell & Tissue Staining Kit (red; Catalog # CTS003) and counterstained with hematoxylin (blue). View our protocol for Chromogenic IHC Staining of Paraffin-embedded Tissue Sections.
 View Larger
View Larger
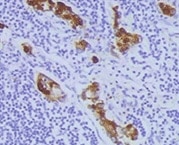
MMP‑1 in Human Ovarian Array. MMP-1 was detected in immersion fixed paraffin-embedded sections of human ovarian array using Mouse Anti-Human MMP-1 Monoclonal Antibody (Catalog # MAB901) at 25 µg/mL overnight at 4 °C. Tissue was stained using the Anti-Mouse HRP-DAB Cell & Tissue Staining Kit (brown; Catalog # CTS002) and counterstained with hematoxylin (blue). Lower panel shows a lack of labeling if primary antibodies are omitted and tissue is stained only with secondary antibody followed by incubation with detection reagents. View our protocol for Chromogenic IHC Staining of Paraffin-embedded Tissue Sections.
 View Larger
View Larger
Western Blot Shows Human MMP‑1 Specificity by Using Knockout Cell Line. Western blot shows lysates of PC-3 human prostate cancer parental cell line and MMP-1 knockout PC-3 cell line (KO). PVDF membrane was probed with 2 µg/mL of Mouse Anti-Human MMP-1 Monoclonal Antibody (Catalog # MAB901) followed by HRP-conjugated Anti-Mouse IgG Secondary Antibody (Catalog # HAF018). A specific band was detected for MMP-1 at approximately 50 kDa (as indicated) in the parental PC-3 cell line, but is not detectable in knockout PC-3 cell line. GAPDH (Catalog # AF5718) is shown as a loading control. This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 1.
 View Larger
View Larger
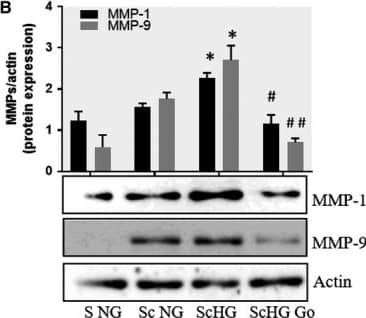
Detection of Human MMP-1 by Western Blot Inhibition of PKC alpha decreases the protein expression of MMP‐1 and MMP‐9 induced by cell cross‐talk in SMC. A, Evaluation of PKC alpha activation in control SMC (S NG) vs SMC previously co‐cultured with MAC in HG (Sc HG). Note that, the phosphorylated form of PKC alpha is significantly increased in SMC that were before exposed to MAC in HG conditions. Silencing of CCR2 or p65 decreases the phosphorylation of PKC alpha to control levels. B, Effect of PKC alpha inhibitor – Go 6976 on the MMP‐1 and MMP‐9 protein expression induced by SMC‐MAC cross‐talk. S NG – control SMC, Sc NG – SMC which were co‐cultured with MAC in normal glucose, Sc HG ‐ SMC which were co‐cultured with MAC in high glucose, Sc HGGo ‐ SMC which were co‐cultured with MAC in high glucose in presence of 1 μmol/L Go 6976. n = 3, *P < .05, **P <.01, #P <.05, ##P <.01 Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/29992758), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
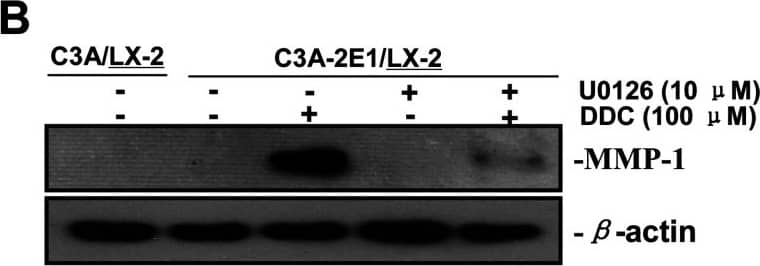
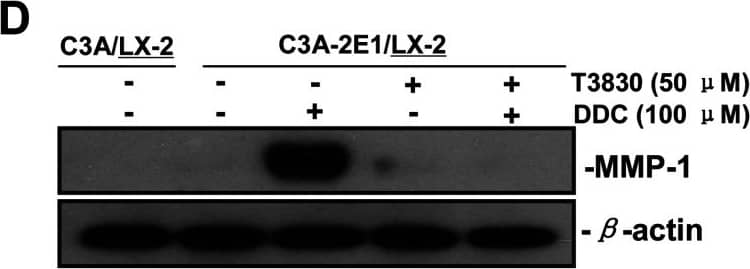
Detection of Human MMP-1 by Western Blot DDC up-regulates MMP-1 through ERK1/2 and Akt activation(A) Co-cultures were treated with 100 μM DDC for the indicated time. Phosphorylation of ERK1/2, p38 and Akt were determined by Western blotting analysis. The corresponding non-phosphorylated ERK1/2, p38, Akt and beta -actin were used for protein loading control. (B) Co-cultures were treated with or without 100 μM DDC for 24 h in the presence or absence of ERK1/2 inhibitor (U0126, 10 μM). MMP-1 protein levels were analysed by Western blotting. (C) Co-cultures were treated with or without 100 μM DDC for 24 h in the presence or absence of p38 inhibitor (SB203580, 10 μM). MMP-1 protein levels were analysed by Western blotting. (D) Co-cultures were treated with or without 100 μM DDC for 24 h in the presence or absence of Akt inhibitor (T3830, 50 μM). MMP-1 protein levels were analysed by Western blotting. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/23577625), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
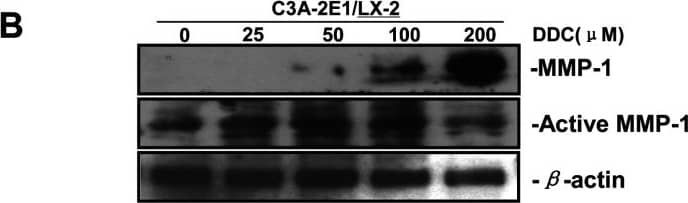
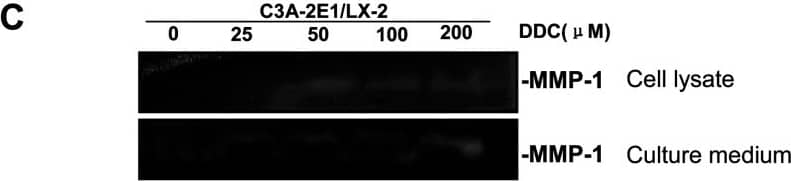
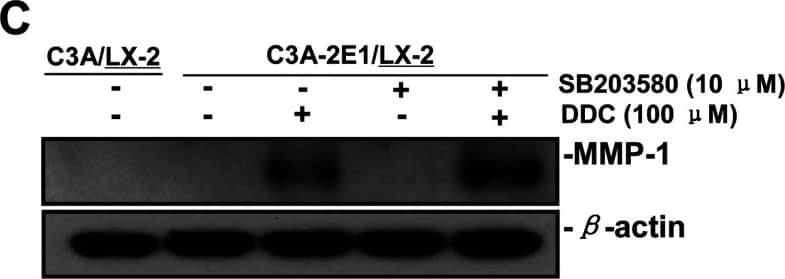
Detection of Human MMP-1 by Western Blot DDC up-regulates MMP-1 expression in a dose-dependent manner and lowered collagen I in LX-2 cellsCo-culture systems were treated with 25, 50, 100 and 200 μM DDC for 24 h. LX-2 cells and the culture medium were collected and analysed. (A) Quantitative RT-PCR results of MMP-1 expression in LX-2 cells. The results were normalized with beta -actin and expressed as fold increase relative to the control in each experiment. (B) 20 μg aliquots of total protein extracts from LX-2 cells were subjected to Western blotting analysis with anti-MMP-1 antibody (MAB 901 and MAB 3223 for active form only) and beta -actin antibody as a loading control, as described in the Materials and Methods section. (C) 30 μg of LX-2 lysates or 20 μl aliquots of 10-fold concentrated culture medium with 5×loading buffer were assayed by Casein Zymography for MMP-1 activity. (D) Levels of collagen I in LX-2 cells were assayed by western blotting. (E) Levels of collagen I in the culture medium were assayed by ELISA. *P<0.05 compared with untreated C3A-2E1/LX-2. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/23577625), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human MMP-1 by Western Blot DDC up-regulates MMP-1 through ERK1/2 and Akt activation(A) Co-cultures were treated with 100 μM DDC for the indicated time. Phosphorylation of ERK1/2, p38 and Akt were determined by Western blotting analysis. The corresponding non-phosphorylated ERK1/2, p38, Akt and beta -actin were used for protein loading control. (B) Co-cultures were treated with or without 100 μM DDC for 24 h in the presence or absence of ERK1/2 inhibitor (U0126, 10 μM). MMP-1 protein levels were analysed by Western blotting. (C) Co-cultures were treated with or without 100 μM DDC for 24 h in the presence or absence of p38 inhibitor (SB203580, 10 μM). MMP-1 protein levels were analysed by Western blotting. (D) Co-cultures were treated with or without 100 μM DDC for 24 h in the presence or absence of Akt inhibitor (T3830, 50 μM). MMP-1 protein levels were analysed by Western blotting. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/23577625), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human MMP-1 by Western Blot DDC up-regulates MMP-1 expression in a dose-dependent manner and lowered collagen I in LX-2 cellsCo-culture systems were treated with 25, 50, 100 and 200 μM DDC for 24 h. LX-2 cells and the culture medium were collected and analysed. (A) Quantitative RT-PCR results of MMP-1 expression in LX-2 cells. The results were normalized with beta -actin and expressed as fold increase relative to the control in each experiment. (B) 20 μg aliquots of total protein extracts from LX-2 cells were subjected to Western blotting analysis with anti-MMP-1 antibody (MAB 901 and MAB 3223 for active form only) and beta -actin antibody as a loading control, as described in the Materials and Methods section. (C) 30 μg of LX-2 lysates or 20 μl aliquots of 10-fold concentrated culture medium with 5×loading buffer were assayed by Casein Zymography for MMP-1 activity. (D) Levels of collagen I in LX-2 cells were assayed by western blotting. (E) Levels of collagen I in the culture medium were assayed by ELISA. *P<0.05 compared with untreated C3A-2E1/LX-2. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/23577625), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
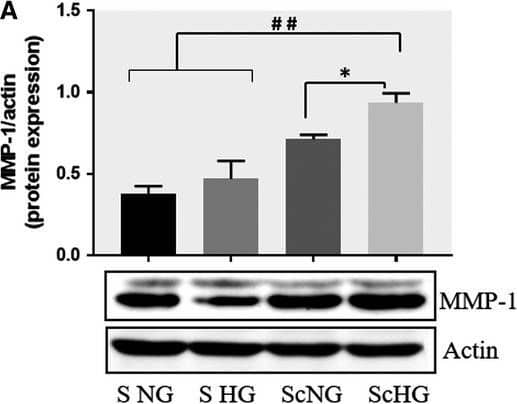
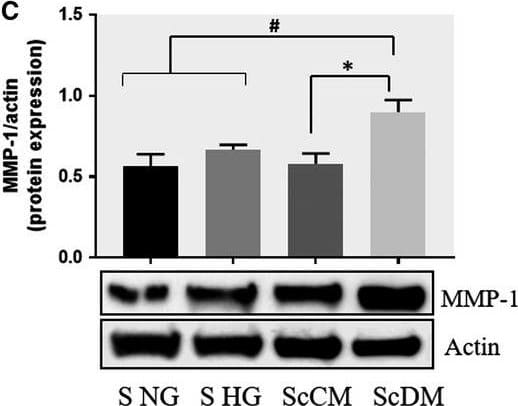
Detection of Human MMP-1 by Western Blot Co‐culture of SMC and MAC in high glucose conditions (24 h) induces a significant increase in the protein expression of MMP‐1 and MMP‐9. A, B: Protein expression of MMP‐1 (A) and MMP‐9 (B) was assessed in SMC after co‐culture with THP‐derived macrophages and evaluated by Western blot assay. C, D: Protein expression of MMP‐1 (C) and MMP‐9 (D) in SMC after co‐culture with human macrophages derived from freshly isolated monocytes from control subjects (CM) or diabetic patients with ACS (DM). S NG –control SMC, S HG – SMC exposed to HG, Sc NG or Sc CM – SMC which were co‐cultured with MAC in normal glucose, Sc HG or Sc DM ‐ SMC which were co‐cultured with MAC in high glucose. n = 3, *P < .05, **P < .01, ***P < .001, #P <.05, ##P <.01, ###P <.001 Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/29992758), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human MMP-1 by Western Blot Co‐culture of SMC and MAC in high glucose conditions (24 h) induces a significant increase in the protein expression of MMP‐1 and MMP‐9. A, B: Protein expression of MMP‐1 (A) and MMP‐9 (B) was assessed in SMC after co‐culture with THP‐derived macrophages and evaluated by Western blot assay. C, D: Protein expression of MMP‐1 (C) and MMP‐9 (D) in SMC after co‐culture with human macrophages derived from freshly isolated monocytes from control subjects (CM) or diabetic patients with ACS (DM). S NG –control SMC, S HG – SMC exposed to HG, Sc NG or Sc CM – SMC which were co‐cultured with MAC in normal glucose, Sc HG or Sc DM ‐ SMC which were co‐cultured with MAC in high glucose. n = 3, *P < .05, **P < .01, ***P < .001, #P <.05, ##P <.01, ###P <.001 Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/29992758), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human MMP-1 by Western Blot DDC up-regulates MMP-1 through ERK1/2 and Akt activation(A) Co-cultures were treated with 100 μM DDC for the indicated time. Phosphorylation of ERK1/2, p38 and Akt were determined by Western blotting analysis. The corresponding non-phosphorylated ERK1/2, p38, Akt and beta -actin were used for protein loading control. (B) Co-cultures were treated with or without 100 μM DDC for 24 h in the presence or absence of ERK1/2 inhibitor (U0126, 10 μM). MMP-1 protein levels were analysed by Western blotting. (C) Co-cultures were treated with or without 100 μM DDC for 24 h in the presence or absence of p38 inhibitor (SB203580, 10 μM). MMP-1 protein levels were analysed by Western blotting. (D) Co-cultures were treated with or without 100 μM DDC for 24 h in the presence or absence of Akt inhibitor (T3830, 50 μM). MMP-1 protein levels were analysed by Western blotting. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/23577625), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human MMP-1 by Western Blot Effect of CCR2 and p65 silencing in the regulation of MMP‐1 and MMP‐9 expression in SMC. Protein expression of MMP‐1 (A) and MMP‐9 (B) determined by Western blot in control SMC (S NG), SMC which were co‐cultured with MAC in HG conditions (Sc HG), and SMC subjected to silencing of CCR‐2 and p65 and co‐cultured with MAC in HG conditions (Cneg, p65, CCR2 siRNA). Note that silencing of CCR2 or p65 decreases significantly the protein expression of MMP‐1 and MMP‐9 induced by SMC‐MAC co‐culture in high glucose conditions. n = 3, *P < .05, #P < .05 Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/29992758), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human MMP-1 by Western Blot DDC up-regulates MMP-1 expression in an H2O2-associated mannerCo-cultures were treated with 100 μM DDC, 2000 Units catalase, 25 μM vitamin E and 5μM H2O2 combined with 100 μM DDC for 24 h. Cell lysates from the LX-2 cells were collected and analysed. (A) 20 μg aliquots of total protein extracts from LX-2 cells were subjected to Western blotting analysis with anti-MMP-1 antibody (MAB 901) and beta -actin antibody as a loading control, as described in the Materials and Methods section. (B) Levels of collagen I in LX-2 cells were assayed by Western blotting. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/23577625), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human MMP-1 by Western Blot DDC up-regulates MMP-1 expression in LX-2 cellsCo-culture systems were treated with 100 μM DDC for 24 h. LX-2 cells and the culture medium were collected and analysed. (A) Quantitative RT-PCR results of MMP-1 expression in LX-2 cells. The results were normalized with beta -actin and expressed as fold increase relative to the control in each experiment. (B) 20 μg aliquots of total protein extracts from LX-2 cells were subjected to western blotting analysis with anti-MMP-1 antibody (MAB 901) and beta -actin antibody as a loading control, as described in the Materials and Methods section. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/23577625), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
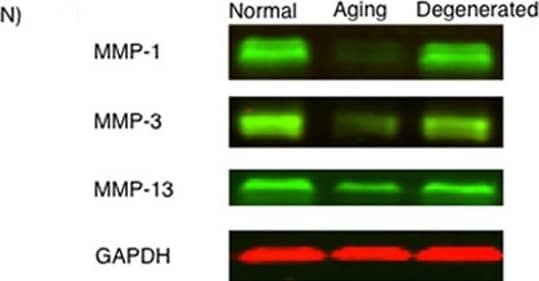
Detection of MMP-1 by Western Blot MMP-1, MMP-3, and MMP-13 expression. (A-D) MMP-1; (E-H) MMP-3; (I-L) MMP-13 (A,E,I) ACL from young normal knee; (B,F,J) ACL from aging knee; (C,G,K) fibroblast-like cell aggregates in the degenerated ACL; (D,H,L) chondrocyte-like cell aggregates in the degenerated ACL. (Original magnification ×40). The graph (M) represents the percentage (mean ± SEM) of MMP-1-, MMP-3-, and MMP-13-positive cells in each group. **P < 0.01. (N) Western blotting for MMP-1, MMP-3, and MMP-13 (n = 3 for each category). Image collected and cropped by CiteAb from the following open publication (https://arthritis-research.biomedcentral.com/articles/10.1186/ar4165), licensed under a CC-BY license. Not internally tested by R&D Systems.
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: MMP-1
Matrix metalloproteinases are a family of zinc and calcium dependent endopeptidases with the combined ability to degrade all the components of the extracellular matrix. MMP-1 (interstitial collagenase), can degrade a broad range of substrates including types I, II, III, VII, VIII, and X collagens as well as casein, gelatin,
alpha ‑1 antitrypsin, myelin basic protein, L-Selectin, pro-TNF, IL-1 beta, IGF-BP3, IGF-BP5, pro MMP-2 and pro MMP-9. A significant role of MMP-1 is the degradation of fibrillar collagens in extracellular matrix remodeling, characterized by the cleavage of the interstitial collagen triple helix into ¾, ¼ fragments. However, as the list of substrates above illustrates, the role of MMP-1 is more diverse than originally envisaged, and may involve enzyme cascades, cytokine regulation and cell surface molecule modulation. MMP-1 is expressed by fibroblasts, keratinocytes, endothelial cells, monocytes and macrophages. Structurally, MMP-1 may be divided into several distinct domains; a pro-domain which is cleaved upon activation; a catalytic domain containing the zinc binding site; a short hinge region and a carboxyl terminal (hemopexin-like) domain.
- Cawston, T.E. (2004) in Interstitial Collagenase. Barrett, A.J. et al. (eds): Handbook of Proteolytic Enzymes, San Diego: Academic Press, p. 472.
Product Datasheets
Citations for Human MMP-1 Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
40
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Redox-sensitive gene-regulatory events controlling aberrant matrix metalloproteinase-1 expression
Authors: Toni R. Bartling, Sita Subbaram, Ryan R. Clark, Akshaya Chandrasekaran, Supriya Kar, J. Andres Andres Melendez
Free Radical Biology and Medicine
-
Cooperative Action of Oxidized Low-Density Lipoproteins and Neutrophils on Endothelial Inflammatory Responses Through Neutrophil Extracellular Trap Formation
Authors: Takashi Obama, Hitomi Ohinata, Takashi Takaki, Sanju Iwamoto, Naoko Sawada, Toshihiro Aiuchi et al.
Frontiers in Immunology
-
Chlamydia pneumoniae induces expression of pro-atherogenic factors through activation of the lectin-like oxidized LDL receptor-1
Authors: Lee Ann Campbell, Amy W. Lee, Michael E. Rosenfeld, Cho-chou Kuo
Pathogens and Disease
-
Mapping Proteolytic Processing in the Secretome of Gastric Cancer-Associated Myofibroblasts Reveals Activation of MMP-1, MMP-2, and MMP-3
Authors: Christopher Holmberg, Bart Ghesquière, Francis Impens, Kris Gevaert, J. Dinesh Kumar, Nicole Cash et al.
Journal of Proteome Research
-
Platelet?rich plasma promotes the migration and invasion of synovial fibroblasts in patients with rheumatoid arthritis
Mol Med Rep, 2016-07-11;0(0):.
-
Blocking TNF alpha attenuates progressive cartilage matrix degradation in inflammatory arthritis
Authors: Jinsung Park, Hyosun Park, Young Lim Lee, Subin Weon, Yong-Gil Kim, Jae-Hyuk Yang et al.
Experimental and Therapeutic Medicine
-
Elevated Serum Gastrin Is Associated with Melanoma Progression: Putative Role in Increased Migration and Invasion of Melanoma Cells
Authors: Varga, AJ;Nemeth, IB;Kemeny, L;Varga, J;Tiszlavicz, L;Kumar, D;Dodd, S;Simpson, AWM;Buknicz, T;Beynon, R;Simpson, D;Krenacs, T;Dockray, GJ;Varro, A;
International journal of molecular sciences
Species: Human
Sample Types: Cell Culture Supernates
Applications: Western Blot -
Improved contractile potential in detrusor microtissues from pediatric patients with end stage lower urinary tract dysfunction
Authors: Gerwinn T, Salemi S, Schori LJ et al.
Frontiers in Cell and Developmental Biology
-
Effect of SPARC Suppression in Mice, Perfused Human Anterior Segments, and Trabecular Meshwork Cells
Authors: WW MacDonald, SS Swaminatha, JY Heo, A Castillejo, J Hsueh, BJ Liu, D Jo, A Du, H Lee, MH Kang, DJ Rhee
Investigative Ophthalmology & Visual Science, 2022-06-01;63(6):8.
Species: Human
Sample Types: Tissue Homogenates
Applications: Western Blot -
Oncogenes overexpressed in metastatic oral cancers from patients with pain: potential pain mediators released in exosomes
Authors: A Bhattachar, MN Janal, R Veeramacha, I Dolgalev, Z Dubeykovsk, NH Tu, H Kim, S Zhang, AK Wu, M Hagiwara, AR Kerr, MD DeLacure, BL Schmidt, DG Albertson
Sci Rep, 2020-09-07;10(1):14724.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
The aryl hydrocarbon receptor pathway controls matrix metalloproteinase-1 and collagen levels in human orbital fibroblasts
Authors: E Roztocil, CL Hammond, MO Gonzalez, SE Feldon, CF Woeller
Sci Rep, 2020-05-21;10(1):8477.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Protective effects of Camellia japonica flower extract against urban air pollutants
Authors: M Kim, D Son, S Shin, D Park, S Byun, E Jung
BMC Complement Altern Med, 2019-01-28;19(1):30.
Species: Human
Sample Types: Whole Tissue
Applications: IHC-P -
Inhibitory Effect of Opuntia humifusa Fruit Water Extract on Solar Ultraviolet-Induced MMP-1 Expression
Authors: AR Han, TG Lim, YR Song, M Jang, YK Rhee, HD Hong, MH Kim, HJ Kim, CW Cho
Int J Mol Sci, 2018-08-24;19(9):.
Species: Human
Sample Types: Whole Tissue
Applications: IHC-P -
The expression of MMP-1 and MMP-9 is up-regulated by smooth muscle cells after their cross-talk with macrophages in high glucose conditions
Authors: RD Macarie, M Vadana, L Ciortan, MM Tucureanu, A Ciobanu, D Vinereanu, I Manduteanu, M Simionescu, E Butoi
J. Cell. Mol. Med., 2018-07-10;0(0):.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Co-treatment of TGF-?3 and BMP7 is superior in stimulating chondrocyte redifferentiation in both hypoxia and normoxia compared to single treatments
Authors: X Huang, L Zhong, JN Post, M Karperien
Sci Rep, 2018-07-06;8(1):10251.
Species: Human
Sample Types: Cell Culture Supernates
Applications: ELISA Development -
The Effects of the WNT-Signaling Modulators BIO and PKF118-310 on the Chondrogenic Differentiation of Human Mesenchymal Stem Cells
Authors: X Huang, L Zhong, J Hendriks, JN Post, M Karperien
Int J Mol Sci, 2018-02-13;19(2):.
Species: Human
Sample Types: Cell Culture Supernates
Applications: ELISA Development -
Matrix metalloproteinase-9 activity and a downregulated Hedgehog pathway impair blood-brain barrier function in an in vitro model of CNS tuberculosis
Authors: S Brilha, CWM Ong, B Weksler, N Romero, PO Couraud, JS Friedland
Sci Rep, 2017-11-22;7(1):16031.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
MLK3 regulates FRA-1 and MMPs to drive invasion and transendothelial migration in triple-negative breast cancer cells
Authors: C Rattanasin, BJ Llewellyn, SE Conrad, KA Gallo
Oncogenesis, 2017-06-12;6(6):e345.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
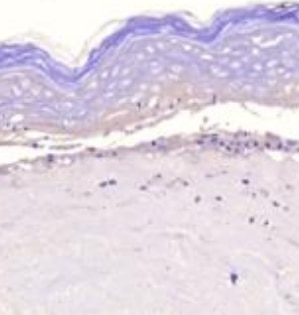
Tumor necrosis factor-alpha-accelerated degradation of type I collagen in human skin is associated with elevated matrix metalloproteinase (MMP)-1 and MMP-3 ex vivo.
Authors: Agren M, Schnabel R, Christensen L, Mirastschijski U
Eur J Cell Biol, 2014-10-23;94(1):12-21.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
MMP-1 and Pro-MMP-10 as potential urinary pharmacodynamic biomarkers of FGFR3-targeted therapy in patients with bladder cancer.
Authors: Du X, Lin B, Wang Q, Li H, Ingalla E, Tien J, Rooney I, Ashkenazi A, Penuel E, Qing J
Clin Cancer Res, 2014-10-17;20(24):6324-35.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Myoferlin depletion in breast cancer cells promotes mesenchymal to epithelial shape change and stalls invasion.
Authors: Li R, Ackerman WE, Mihai C
PLoS ONE, 2012-06-27;7(6):e39766.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Chondrocytes from patients with osteoarthritis express typical extracellular matrix molecules once grown onto a three-dimensional hyaluronan-based scaffold.
Authors: Cavallo C, Desando G, Facchini A, Grigolo B
J Biomed Mater Res A, 2010-04-01;93(1):86-95.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Effect of bimatoprost, latanoprost, and unoprostone on matrix metalloproteinases and their inhibitors in human ciliary body smooth muscle cells.
Authors: Ooi YH, Oh DJ, Rhee DJ
Invest. Ophthalmol. Vis. Sci., 2009-05-14;50(11):5259-65.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Tissue inhibitors of matrix metalloproteinases in platelets and megakaryocytes: a novel organization for these secreted proteins.
Authors: Villeneuve J, Block A, Le Bousse-Kerdiles MC, Lepreux S, Nurden P, Ripoche J, Nurden AT
Exp. Hematol., 2009-05-03;37(7):849-56.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Matrix metalloproteinase activity in pediatric acute lung injury.
Authors: Kong MY, Gaggar A, Li Y
Int J Med Sci, 2008-12-16;6(1):9-17.
Species: Human
Sample Types: Tissue Secretion
Applications: Western Blot -
The association of Sam68 with Vav1 contributes to tumorigenesis.
Authors: Lazer G, Pe'er L, Schapira V, Richard S, Katzav S
Cell. Signal., 2007-08-08;19(12):2479-86.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Interleukin-1beta induced activation of nuclear factor-kappab can be inhibited by novel pharmacological agents in osteoarthritis.
Authors: Lauder SN, Carty SM, Carpenter CE
Rheumatology (Oxford), 2007-01-11;46(5):752-8.
Species: Human
Sample Types: Cell Culture Supernates
Applications: ELISA Development -
Gingival fibroblasts inhibit MMP-1 and MMP-3 activities in an ex-vivo artery model.
Authors: Naveau A, Reinald N, Fournier B
Connect. Tissue Res., 2007-01-01;48(6):300-8.
Species: Human
Sample Types: Whole Cells
Applications: Dot Blot -
Control of matrix metalloproteinase production in human intestinal fibroblasts by interleukin 21.
Authors: Monteleone G, Caruso R, Fina D, Peluso I, Gioia V, Stolfi C, Fantini MC, Caprioli F, Tersigni R, Alessandroni L, MacDonald TT, Pallone F
Gut, 2006-05-08;55(12):1774-80.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
c-Kit immunophenotyping and metalloproteinase expression profiles of mast cells in interstitial lung diseases.
Authors: Edwards ST, Cruz AC, Donnelly S, Dazin PF, Schulman ES, Jones KD, Wolters PJ, Hoopes C, Dolganov GM, Fang KC
J. Pathol., 2005-07-01;206(3):279-90.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Effects of autologous platelet‑rich plasma injections on facial skin rejuvenation
Authors: Rina Du, Tiechi Lei
Experimental and Therapeutic Medicine
-
Cellular and extracellular matrix changes in anterior cruciate ligaments during human knee aging and osteoarthritis
Authors: Akihiko Hasegawa, Hiroyuki Nakahara, Mitsuo Kinoshita, Hiroshi Asahara, James Koziol, Martin K Lotz
Arthritis Research & Therapy
-
Silencing microRNA-330-5p increases MMP1 expression and promotes an invasive phenotype in oesophageal adenocarcinoma
Authors: Becky A. S. Bibby, Cecelia S. Miranda, John V. Reynolds, Christopher J. Cawthorne, Stephen G. Maher
BMC Cancer
-
Epiregulin contributes to breast tumorigenesis through regulating matrix metalloproteinase 1 and promoting cell survival
Authors: Mariya Farooqui, Laura R. Bohrer, Nicholas J. Brady, Pavlina Chuntova, Sarah E. Kemp, C. Taylor Wardwell et al.
Molecular Cancer
-
Cabozantinib (XL184) and R428 (BGB324) Inhibit the Growth of Esophageal Squamous Cell Carcinoma (ESCC)
Authors: Pei-Wen Yang, Yu-Cheng Liu, Ya-Han Chang, Ching-Ching Lin, Pei-Ming Huang, Kuo-Tai Hua et al.
Frontiers in Oncology
-
Matrix Metalloproteinase-1 and Acid Phosphatase in the Degradation of the Lamina Propria of Eruptive Pathway of Rat Molars
Authors: José Paulo de Pizzol Júnior, Estela Sasso-Cerri, Paulo Sérgio Cerri
Cells
-
Improved contractile potential in detrusor microtissues from pediatric patients with end stage lower urinary tract dysfunction
Authors: Gerwinn T, Salemi S, Schori LJ et al.
Frontiers in Cell and Developmental Biology
-
The anti-wrinkles properties of sodium acetylated hyaluronate
Authors: Meunier M, Scandolera A, Chapuis E Et al.
Journal of cosmetic dermatology
-
Reactive oxygen species control senescence-associated matrix metalloproteinase-1 through c-Jun-N-terminal kinase
Authors: Jaya Dasgupta, Supriya Kar, Rong Liu, Joy Joseph, Balaraman Kalyanaraman, S. James Remington et al.
Journal of Cellular Physiology
-
Suppressed Expression but Not Activity of Collagenases MMP-1 and MMP-13 in Human Renal Carcinoma
Authors: Grzegorz Młynarczyk, Jacek Kudelski, Barbara Darewicz, Marta Bruczko-Goralewska, Lech Romanowicz
Pathobiology
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Human MMP-1 Antibody
Average Rating: 4.6 (Based on 5 Reviews)
Have you used Human MMP-1 Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by: