Human IL-2 DuoSet ELISA Summary
* Provided that the recommended microplates, buffers, diluents, substrates and solutions are used, and the assay is run as summarized in the Assay Procedure provided.
This DuoSet ELISA Development kit contains the basic components required for the development of sandwich ELISAs to measure natural and recombinant human IL-2. The suggested diluent is suitable for the analysis of most cell culture supernate samples. Diluents for complex matrices, such as serum and plasma, should be evaluated prior to use in this DuoSet.
Product Features
- Optimized capture and detection antibody pairings with recommended concentrations save lengthy development time
- Development protocols are provided to guide further assay optimization
- Assay can be customized to your specific needs
- Economical alternative to complete kits
Kit Content
- Capture Antibody
- Detection Antibody
- Recombinant Standard
- Streptavidin conjugated to horseradish-peroxidase (Streptavidin-HRP)
Other Reagents Required
DuoSet Ancillary Reagent Kit 2 (5 plates): (Catalog # DY008) containing 96 well microplates, plate sealers, substrate solution, stop solution, plate coating buffer (PBS), wash buffer, and Reagent Diluent Concentrate 2.
The components listed above may be purchased separately:
PBS: (Catalog # DY006), or 137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4, pH 7.2 - 7.4, 0.2 µm filtered
Wash Buffer: (Catalog # WA126), or 0.05% Tween® 20 in PBS, pH 7.2-7.4
Block Buffer: (Catalog # DY995), or 1% BSA in PBS, pH 7.2-7.4, 0.2 µm filtered
Reagent Diluent: 0.1% BSA, 0.05% Tween 20 in Tris-buffered Saline (20 mM Trizma base, 150 mM NaCI) pH 7.2-7.4, 0.2 μm filtered
Substrate Solution: 1:1 mixture of Color Reagent A (H2O2) and Color Reagent B (Tetramethylbenzidine) (Catalog # DY999)
Stop Solution: 2 N H2SO4 (Catalog # DY994)
Microplates: R&D Systems (Catalog # DY990)
Plate Sealers: ELISA Plate Sealers (Catalog # DY992)
Scientific Data
Product Datasheets
Preparation and Storage
Background: IL-2
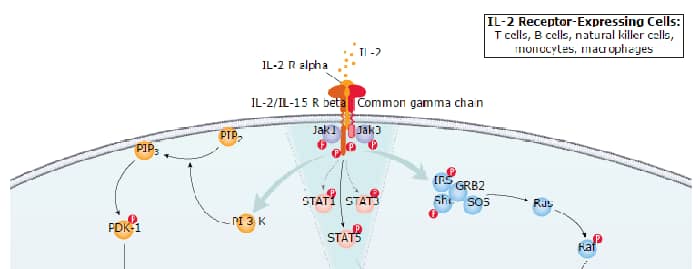
Interleukin 2 (IL-2), also known as T cell growth factor (TCGF), is a 15-18 kDa variably glycosylated alpha -helical polypeptide that is a member of the Common gamma Chain ( gamma c) cytokine family (1-4). It exists as a monomer and has a notably short half-life (< 30 minutes) (1). Human IL-2 is synthesized as a 153 amino acid (aa) precursor that contains a 20 aa signal sequence plus a 133 aa mature region (5, 6). The mature region is alpha -helical in nature, and contains one utilized O-linked glycosylation site at Thr3 plus three cysteines, two of which form an intrachain disulfide bond that is essential for activity (7). Mature human IL-2 shares 73%, 66%, 78% and 97% aa identity with canine, rat, feline and rhesus monkey IL-2, respectively. Although human IL-2 shares only approximately 60% aa identity with the highly polymorphic mouse IL-2, human IL-2 is known to be active on mouse IL-2 responsive cells. Cells reported to secrete IL-2 include gamma δ T cells (8), activated conventional CD4+ and CD8+ T cells (1, 9), neurons (10, 11), microglia (12), and hematopoietic stem cells (13).
Assay Procedure
GENERAL ELISA PROTOCOL
Plate Preparation
- Dilute the Capture Antibody to the working concentration in PBS without carrier protein. Immediately coat a 96-well microplate with 100 μL per well of the diluted Capture Antibody. Seal the plate and incubate overnight at room temperature.
- Aspirate each well and wash with Wash Buffer, repeating the process two times for a total of three washes. Wash by filling each well with Wash Buffer (400 μL) using a squirt bottle, manifold dispenser, or autowasher. Complete removal of liquid at each step is essential for good performance. After the last wash, remove any remaining Wash Buffer by aspirating or by inverting the plate and blotting it against clean paper towels.
- Block plates by adding 300 μL of Block Buffer to each well. Incubate at room temperature for a minimum of 1 hour.
- Repeat the aspiration/wash as in step 2. The plates are now ready for sample addition.
Assay Procedure
- Add 100 μL of sample or standards in Reagent Diluent, or an appropriate diluent, per well. Cover with an adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the Detection Antibody, diluted in Reagent Diluent, to each well. Cover with a new adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the working dilution of Streptavidin-HRP to each well. Cover the plate and incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Repeat the aspiration/wash as in step 2.
- Add 100 μL of Substrate Solution to each well. Incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Add 50 μL of Stop Solution to each well. Gently tap the plate to ensure thorough mixing.
- Determine the optical density of each well immediately, using a microplate reader set to 450 nm. If wavelength correction is available, set to 540 nm or 570 nm. If wavelength correction is not available, subtract readings at 540 nm or 570 nm from the readings at 450 nm. This subtraction will correct for optical imperfections in the plate. Readings made directly at 450 nm without correction may be higher and less accurate.
Citations for Human IL-2 DuoSet ELISA
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
74
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Paired CRISPR screens to map gene regulation in cis and trans
Authors: Xue, X;Gajic, ZZ;Caragine, CM;Legut, M;Walker, C;Kim, JYS;Wang, X;Yan, RE;Wessels, HH;Lu, C;Bapodra, N;Gürsoy, G;Sanjana, NE;
bioRxiv : the preprint server for biology
Species: Human
Sample Types: Cell Culture Supernates
-
Preclinical comparison of prolgolimab, pembrolizumab and nivolumab
Authors: Aleksandr, G;Andrei, V;Maria, P;Iana, S;Aleksandr, D;Anna, Z;Daria, Z;Aleksei, A;Mikhail, S;Evgeny, I;Valery, S;Pavel, I;
Scientific reports
Species: Human
Sample Types: Cell Culture Supernates
-
CAR T-cell-mediated delivery of bispecific innate immune cell engagers for neuroblastoma
Authors: Pascual-Pasto, G;McIntyre, B;Hines, MG;Giudice, AM;Garcia-Gerique, L;Hoffmann, J;Mishra, P;Matlaga, S;Lombardi, S;Shraim, R;Schürch, PM;Yarmarkovich, M;Hofmann, TJ;Alikarami, F;Martinez, D;Tsang, M;Gil-de-Gómez, L;Spear, TT;Bernt, KM;Wolpaw, AJ;Dimitrov, DS;Li, W;Bosse, KR;
Nature communications
Species: Human
Sample Types: Cell Culture Supernates
-
Cognitive function is associated with performance in time up and go test and with leptin blood levels in community-dwelling older women
Authors: da Costa Teixeira, LA;Soares, LA;Lima, LP;Avelar, NCP;de Moura, JA;Leopoldino, AAO;Figueiredo, PHS;Parentoni, AN;Mendonça, VA;Lacerda, ACR;
Scientific reports
Species: Human
Sample Types: Plasma
-
The strong inverse association between plasma concentrations of soluble tumor necrosis factor receptors type 1 with adiponectin/leptin ratio in older women
Authors: Augusto da Costa Teixeira, L;Rocha-Vieira, E;Aparecida Soares, L;Mota de Oliveira, F;Aparecida Oliveira Leopoldino, A;Netto Parentoni, A;Amaral Mendonça, V;Cristina Rodrigues Lacerda, A;
Cytokine
Species: Human
Sample Types: Plasma
-
A Comparison of the Antitumor Efficacy of Novel Multi-Specific Tribodies with Combinations of Approved Immunomodulatory Antibodies
Authors: Manna, L;Rapuano Lembo, R;Yoshioka, A;Nakamura, K;Passariello, M;De Lorenzo, C;
Cancers
Species: Human
Sample Types: Cell Culture Supernatents
-
Serum interleukin-6, procalcitonin, and C-reactive protein at hospital admission can identify patients at low risk for severe COVID-19 progression
Authors: Zobel, CM;Wenzel, W;Krüger, JP;Baumgarten, U;Wagelöhner, T;Neumann, N;Foroutan, B;Müller, R;Müller, A;Rauschning, D;Schü beta ler, M;Scheit, L;Weinreich, F;Oltmanns, K;Keidel, F;Koch, M;Spethmann, S;Schreiner, M;
Frontiers in microbiology
Species: Human
Sample Types: Serum
-
Clinical Staphylococcus aureus inhibits human T-cell activity through interaction with the PD-1 receptor
Authors: Mellergaard, M;Skovbakke, SL;Jepsen, SD;Panagiotopoulou, N;Hansen, ABR;Tian, W;Lund, A;Høgh, RI;Møller, SH;Guérillot, R;Hayes, AS;Erikstrup, LT;Andresen, L;Peleg, AY;Larsen, AR;Stinear, TP;Handberg, A;Erikstrup, C;Howden, BP;Goletz, S;Frees, D;Skov, S;
mBio
-
Discovery of potent and selective HPK1 inhibitors based on the 2,4-disubstituted pyrimidine scaffold with immune modulatory properties for ameliorating T cell exhaustion
Authors: Zeng, S;Zeng, M;Yuan, S;He, L;Jin, Y;Huang, J;Zhang, M;Yang, M;Pan, Y;Wang, Z;Chen, Y;Xu, X;Huang, W;
Bioorganic chemistry
Species: Human
Sample Types: Cell Culture Supernates
-
Inflammatory biomarkers at different stages of Sarcopenia in older women
Authors: da Costa Teixeira, LA;Avelar, NCP;Peixoto, MFD;Parentoni, AN;Santos, JMD;Pereira, FSM;Danielewicz, AL;Leopoldino, AAO;Costa, SP;Arrieiro, AN;Soares, LA;da Silva Lage, VK;Prates, ACN;Taiar, R;de Carvalho Bastone, A;Oliveira, VC;Oliveira, MX;Costa, HS;Nobre, JNP;Brant, FP;Duarte, TC;Figueiredo, PHS;Mendonça, VA;Lacerda, ACR;
Scientific reports
Species: Human
Sample Types: Plasma
-
T Cells Expressing a Modified FcgammaRI Exert Antibody-Dependent Cytotoxicity and Overcome the Limitations of CAR T-cell Therapy against Solid Tumors
Authors: D Rasoulouni, N Santana-Ma, A Gutwillig, L Farhat-You, L Tal, S Amar, M Milyavsky, SSNA Muddineni, N Solomon, H Shpilt, S Dotan, N Pilpel, C Waskow, M Feinmesser, P Rider, Y Carmi
Cancer Immunology Research, 2023-06-02;0(0):OF1-OF18.
Species: Human
Sample Types: Cell Culture Supernates
-
Characterization of 405B8H3(D-E), a newly engineered high affinity chimeric LAG-3 antibody with potent antitumor activity
Authors: Lan, X;Yang, TTC;Wang, Y;Qu, B;Rong, S;Song, N;
FEBS open bio
Species: Human
Sample Types: Cell Culture Supernates
-
Combinatorial inhibition of Tec kinases BTK and ITK is beneficial in ameliorating murine sclerodermatous chronic graft versus host disease
Authors: Palaniyandi, S;Strattan, E;Kumari, R;Mysinger, M;Hakim, N;Kesler, MV;Apatira, M;Bittencourt, F;Wang, L;Jia, Z;Gururaja, TL;Hill, RJ;Hildebrandt, GC;
Bone marrow transplantation
Species: Human
Sample Types: Cell Culture Supernates
-
Adiponectin Is a Contributing Factor of Low Appendicular Lean Mass in Older Community-Dwelling Women: A Cross-Sectional Study
Authors: LAC Teixeira, JM Dos Santos, AN Parentoni, LP Lima, TC Duarte, FP Brant, CDC Neves, FSM Pereira, NCP Avelar, AL Danielewic, AAO Leopoldino, SP Costa, AN Arrieiro, LA Soares, ACN Prates, JNP Nobre, A de Carvalh, VC de Oliveir, MX Oliveira, PH Scheidt Fi, HS Costa, V Amaral Men, R Taiar, AC Rodrigues
Journal of Clinical Medicine, 2022-12-02;11(23):.
Species: Human
Sample Types: Plasma
-
Co-expression IL-15 receptor alpha with IL-15 reduces toxicity via limiting IL-15 systemic exposure during CAR-T immunotherapy
Authors: Y Zhang, Q Zhuang, F Wang, C Zhang, C Xu, A Gu, WH Zhong, Y Hu, X Zhong
Oncogene, 2022-09-27;20(1):432.
Species: Human
Sample Types: Cell Culture Supernates
-
Generation and Functional Characterization of PLAP CAR-T Cells against Cervical Cancer Cells
Authors: V Yekehfalla, S Pahlavanne, A Sayadmanes, Z Momtahan, B Ma, M Basiri
Biomolecules, 2022-09-14;12(9):.
Species: Human
Sample Types: Cell Culture Supernatants
-
SARS-CoV-2 Spike Does Not Possess Intrinsic Superantigen-like Inflammatory Activity
Authors: C Amormino, V Tedeschi, G Paldino, S Arcieri, MT Fiorillo, A Paiardini, L Tuosto, M Kunkl
Cells, 2022-08-15;11(16):.
Species: Human
Sample Types: Cell Culture Supernates
-
HydrAd: A Helper-Dependent Adenovirus Targeting Multiple Immune Pathways for Cancer Immunotherapy
Authors: A Rosewell S, C Porter, G Biegert, L Jatta, M Suzuki
Oncogene, 2022-06-02;14(11):.
Species: Human
Sample Types: Cell Culture Supernates
-
Novel Bi-Specific Immuno-Modulatory Tribodies Potentiate T Cell Activation and Increase Anti-Tumor Efficacy
Authors: M Passariell, A Yoshioka, K Takahashi, SI Hashimoto, R Rapuano Le, L Manna, K Nakamura, C De Lorenzo
International Journal of Molecular Sciences, 2022-03-23;23(7):.
Species: Human
Sample Types: Cell Culture Supernatents
-
Activation of the Innate Immune Checkpoint CLEC5A on Myeloid Cells in the Absence of Danger Signals Modulates Macrophages' Function but Does Not Trigger the Adaptive T Cell Immune Response
Authors: MJ Tosiek, K Groesser, A Pekcec, M Zwirek, G Murugesan, E Borges
Journal of Immunology Research, 2022-02-25;2022(0):9926305.
Species: Human
Sample Types: Cell Culture Supernates
-
Novel Combinations of Human Immunomodulatory mAbs Lacking Cardiotoxic Effects for Therapy of TNBC
Authors: C Vetrei, M Passariell, G Froechlich, R Rapuano Le, E Sasso, N Zambrano, C De Lorenzo
Cancers, 2021-12-27;14(1):.
Species: Human
Sample Types: Cell Culture Supernates
-
Discovery of a novel anti PD-L1 X TIGIT bispecific antibody for the treatment of solid tumors
Authors: Y Xiao, P Chen, C Luo, Z Xu, X Li, L Liu, L Zhao
Cancer treatment and research communications, 2021-09-27;29(0):100467.
Species: Human
Sample Types: Cell Culture Supernates
-
LFA-1 and Kindlin-3 enable the collaborative transport of SLP-76 microclusters by myosin and dynein motors
Authors: KP Eidell, A Lovy, NR Sylvain, FA Scangarell, HI Muendlein, MJ Ophir, K Nguyen, MC Seminario, SC Bunnell
Journal of Cell Science, 2021-08-27;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
A scDb-based trivalent bispecific antibody for T-cell-mediated killing of HER3-expressing cancer cells
Authors: N Aschmoneit, S Steinlein, L Kühl, O Seifert, RE Kontermann
Scientific Reports, 2021-07-06;11(1):13880.
Species: Human
Sample Types: Cell Culture Supernates
-
Loureirin B Exerts its Immunosuppressive Effects by Inhibiting STIM1/Orai1 and KV1.3 Channels
Authors: S Shi, Q Zhao, C Ke, S Long, F Zhang, X Zhang, Y Li, X Liu, H Hu, S Yin
Frontiers in Pharmacology, 2021-06-25;12(0):685092.
Species: Human
Sample Types: Cell Culture Supernates
-
Immunomodulatory mAbs as Tools to Investigate on Cis-Interaction of PD-1/PD-L1 on Tumor Cells and to Set Up Methods for Early Screening of Safe and Potent Combinatorial Treatments
Authors: C Vetrei, M Passariell, G Froechlich, R Rapuano Le, N Zambrano, C De Lorenzo
Cancers, 2021-06-08;13(12):.
Species: Human
Sample Types: Cell Culture Supernates
-
A Rational Designed Novel Bispecific Antibody for the Treatment of GBM
Authors: R Sun, Y Zhou, L Han, Z Pan, J Chen, H Zong, Y Bian, H Jiang, B Zhang, J Zhu
Biomedicines, 2021-06-03;9(6):.
Species: Human
Sample Types: Cell Culture Supernates
-
T cell receptor-dependent S-acylation of ZAP-70 controls activation of T cells
Authors: R Tewari, B Shayahati, Y Fan, AM Akimzhanov
The Journal of Biological Chemistry, 2021-01-19;0(0):100311.
Species: Human
Sample Types: Cell Culture Supernates
-
Altered plasma levels of &betaC and &gammaC chain cytokines and post-treatment modulation in tuberculous lymphadenitis
Authors: GR Kathamuthu, K Moideen, R Sridhar, D Baskaran, S Babu
Cytokine, 2020-12-17;138(0):155405.
Species: Human
Sample Types: Plasma
-
Aromadendrin Inhibits T Cell Activation via Regulation of Calcium Influx and NFAT Activity
Authors: HS Lee, GS Jeong
Molecules, 2020-10-08;25(19):.
Species: Human
Sample Types: Cell Culture Supernates
-
Lentiviral delivery of combinatorial CAR/CRISPRi circuit into human primary T cells is enhanced by TBK1/IKK? complex inhibitor BX795
Authors: L Li, Y Gao, R Srivastava, W Wang, Q Xiong, Z Fang, A Pelayo, C Denson, A Goswami, R Harari-Ste, Z Yang, L Weng, LS Qi, FM Marincola
Journal of Translational Medicine, 2020-09-23;18(1):363.
Species: Human
Sample Types: Cell Culture Supernates
-
Targeting PD-L1 in non-small cell lung cancer using CAR T cells
Authors: M Liu, X Wang, W Li, X Yu, P Flores-Vil, ZY Xu-Monette, L Li, M Zhang, KH Young, X Ma, Y Li
Oncogenesis, 2020-08-13;9(8):72.
Species: Human
Sample Types: Cell Culture Supernates
-
Isolation of Two Novel Human Anti-CTLA-4 mAbs with Intriguing Biological Properties on Tumor and NK Cells
Authors: M Passariell, C Vetrei, E Sasso, G Froechlich, C Gentile, AM D'Alise, N Zambrano, E Scarselli, A Nicosia, C De Lorenzo
Cancers (Basel), 2020-08-06;12(8):.
Species: Human
Sample Types: Cell Culture Supernates
-
IRAP-dependent endosomal T cell receptor signalling is essential for T cell responses
Authors: I Evnouchido, P Chappert, S Benadda, A Zucchetti, M Weimershau, M Bens, V Caillens, D Koumantou, S Lotersztaj, P van Endert, J Davoust, P Guermonpre, C Hivroz, DA Gross, L Saveanu
Nat Commun, 2020-06-02;11(1):2779.
Species: Human
Sample Types: Cell Culture Supernates
-
Single residue in CD28-costimulated CAR-T cells limits long-term persistence and antitumor durability
Authors: S Guedan, A Madar, V Casado-Med, C Shaw, A Wing, F Liu, RM Young, CH June, AD Posey
J. Clin. Invest., 2020-06-01;130(6):3087-3097.
Species: Human
Sample Types: Cell Culture Supernates
-
Gaps in Study Design for Immune Parameter Research for Latent Tuberculosis Infection: A Systematic Review
Authors: M Herrera, C Vera, Y Keynan, ZV Rueda
J Immunol Res, 2020-04-21;2020(0):8074183.
Species: Human
Sample Types: Plasma
-
Ipilimumab and Its Derived EGFR Aptamer-Based Conjugate Induce Efficient NK Cell Activation against Cancer Cells
Authors: M Passariell, S Camorani, C Vetrei, S Ricci, L Cerchia, C De Lorenzo
Cancers (Basel), 2020-02-01;12(2):.
Species: Human
Sample Types: Cell Culture Supernates
-
Ultrasmall silica nanoparticles directly ligate the T cell receptor complex
Authors: B Vis, RE Hewitt, TP Monie, C Fairbairn, SD Turner, SD Kinrade, JJ Powell
Proc. Natl. Acad. Sci. U.S.A., 2019-12-23;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Type 1 diabetic mellitus patients with increased atherosclerosis risk display decreased CDKN2A/2B/2BAS gene expression in leukocytes
Authors: S Martínez-H, V Sánchez-Ga, A Herrero-Ce, Á Vinué, JT Real, JF Ascaso, DJ Burks, H González-N
J Transl Med, 2019-07-12;17(1):222.
Species: Human
Sample Types: Plasma
-
Quantitative Interactomics in Primary T Cells Provides a Rationale for Concomitant PD-1 and BTLA Coinhibitor Blockade in Cancer Immunotherapy
Authors: J Celis-Guti, P Blattmann, Y Zhai, N Jarmuzynsk, K Ruminski, C Grégoire, Y Ounoughene, F Fiore, R Aebersold, R Roncagalli, M Gstaiger, B Malissen
Cell Rep, 2019-06-11;27(11):3315-3330.e7.
Species: Human
Sample Types: Cell Culture Supernates
-
Role of vimentin in modulating immune cell apoptosis and inflammatory responses in sepsis
Authors: L Su, P Pan, P Yan, Y Long, X Zhou, X Wang, R Zhou, B Wen, L Xie, D Liu
Sci Rep, 2019-04-05;9(1):5747.
Species: Human
Sample Types: Cell Culture Supernates
-
Urinary and serum soluble CD25 complements urinary soluble CD163 to detect active renal anti-neutrophil cytoplasmic autoantibody-associated vasculitis: a cohort study
Authors: GJ Dekkema, WH Abdulahad, T Bijma, SM Moran, L Ryan, MA Little, CA Stegeman, P Heeringa, JF Sanders
Nephrol. Dial. Transplant., 2019-02-01;0(0):.
Species: Human
Sample Types: Serum
-
Sulphamethazine derivatives as immunomodulating agents: New therapeutic strategies for inflammatory diseases
Authors: H Siddiqui, HM Haniffa, A Jabeen, AU -Rahman, MI Choudhary
PLoS ONE, 2018-12-19;13(12):e0208933.
Species: Human
Sample Types: Cell Culture Supernates
-
Modulation of intracellular calcium signaling by microRNA-34a-5p
Authors: C Diener, M Hart, D Alansary, V Poth, B Walch-Rück, J Menegatti, F Grässer, T Fehlmann, S Rheinheime, BA Niemeyer, HP Lenhof, A Keller, E Meese
Cell Death Dis, 2018-09-27;9(10):1008.
Species: Human
Sample Types: Whole Cells
-
Interferon-? converts human microvascular pericytes into negative regulators of alloimmunity through induction of indoleamine 2,3-dioxygenase 1
Authors: R Liu, J Merola, TD Manes, L Qin, GT Tietjen, F López-Girá, V Broecker, C Fang, C Xie, PM Chen, NC Kirkiles-S, D Jane-Wit, JS Pober
JCI Insight, 2018-03-08;3(5):.
Species: Human
Sample Types: Cell Culture Supernates
-
Enhancing CAR T cell persistence through ICOS and 4-1BB costimulation.
Authors: Guedan S, Posey A, Shaw C, Wing A, Da T, Patel P, McGettigan S, Casado-Medrano V, Kawalekar O, Uribe-Herranz M, Song D, Melenhorst J, Lacey S, Scholler J, Keith B, Young R, June C
JCI Insight, 2018-01-11;3(1):.
Species: Human
Sample Types: Cell Culture Supernates
-
One-domain CD4 Fused to Human Anti-CD16 Antibody Domain Mediates Effective Killing of HIV-1-Infected Cells
Authors: W Li, Y Wu, D Kong, H Yang, Y Wang, J Shao, Y Feng, W Chen, L Ma, T Ying, DS Dimitrov
Sci Rep, 2017-08-22;7(1):9130.
Species: Human
Sample Types: Cell Culture Supernates
-
Identification of benzazole compounds that induce HIV-1 transcription
Authors: JD Graci, D Michaels, G Chen, GM Schiralli, S Nodder, M Weetall, GM Karp, Z Gu, JM Colacino, AJ Henderson
PLoS ONE, 2017-06-28;12(6):e0179100.
Species: Human
Sample Types: Cell Culture Supernates
-
Reduced systemic and mycobacterial antigen-stimulated concentrations of IL-1? and IL-18 in tuberculous lymphadenitis
Cytokine, 2016-10-26;90(0):66-72.
Species: Human
Sample Types: Cell Culture Supernates
-
Chemical proteomic map of dimethyl fumarate-sensitive cysteines in primary human T cells
Authors: Benjamin F Cravatt
Sci Signal, 2016-09-13;9(445):rs10.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Rac1-Rab11-FIP3 regulatory hub coordinates vesicle traffic with actin remodeling and T-cell activation
EMBO J, 2016-05-06;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Additive anti-inflammatory effects of corticosteroids and phosphodiesterase-4 inhibitors in COPD CD8 cells.
Authors: Grundy S, Plumb J, Kaur M, Ray D, Singh D
Respir Res, 2016-01-25;17(1):9.
Species: Human
Sample Types: Cell Culture Supernates
-
Characterization of host and microbial determinants in individuals with latent tuberculosis infection using a human granuloma model.
Authors: Guirado E, Mbawuike U, Keiser T, Arcos J, Azad A, Wang S, Schlesinger L
MBio, 2015-02-17;6(1):e02537-14.
Species: Human
Sample Types: Cell Culture Supernates
-
Anti-inflammatory effects of green soybean extract irradiated with visible light.
Authors: Tanaka K, Ohgo Y, Katayanagi Y, Yasui K, Hiramoto S, Ikemoto H, Nakata Y, Miyoshi N, Isemura M, Ohashi N, Imai S
Sci Rep, 2014-04-22;4(0):4732.
Species: Human
Sample Types: Cell Culture Supernates
-
Engagement of SLAMF2/CD48 prolongs the time frame of effective T cell activation by supporting mature dendritic cell survival.
Authors: Kis-Toth K, Tsokos G
J Immunol, 2014-03-26;192(9):4436-42.
Species: Human
Sample Types: Cell Culture Supernates
-
The aryl hydrocarbon receptor is functionally upregulated early in the course ofvhuman T-cell activation.
Authors: Prigent L, Robineau M, Jouneau S, Morzadec C, Louarn L, Vernhet L, Fardel O, Sparfel L
Eur J Immunol, 2014-02-18;44(5):1330-40.
Species: Human
Sample Types: Cell Culture Supernates
-
The tuberculosis vaccine candidate Bacillus Calmette-Guerin DeltaureC::hly coexpressing human interleukin-7 or -18 enhances antigen-specific T cell responses in mice.
Authors: Rao M, Vogelzang A, Kaiser P, Schuerer S, Kaufmann S, Gengenbacher M
PLoS ONE, 2013-11-13;8(11):e78966.
Species: Human
Sample Types: Cell Culture Supernates
-
T-cell engager-armed oncolytic vaccinia virus significantly enhances antitumor therapy.
Authors: Yu F, Wang X, Guo Z, Bartlett D, Gottschalk S, Song X
Mol Ther, 2013-10-17;22(1):102-11.
Species: Human
Sample Types: Cell Culture Supernates
-
Dynamic motile T cells highly respond to the T cell stimulation via PI3K-Akt and NF-kappaB pathways.
Authors: Kim H, Na B, Kwon M, Ko Y, Han W, Jun C
PLoS ONE, 2013-03-26;8(3):e59793.
Species: Human
Sample Types: Cell Culture Supernates
-
Leukocyte function-associated antigen-1/intercellular adhesion molecule-1 interaction induces a novel genetic signature resulting in T-cells refractory to transforming growth factor-beta signaling.
Authors: Verma N, Dempsey E, Long A, Davies A, Barry S, Fallon P, Volkov Y, Kelleher D
J Biol Chem, 2012-06-15;287(32):27204-16.
Species: Human
Sample Types: Cell Culture Supernates
-
Immunoregulation of autocrine prolactin: suppressing the expression of costimulatory molecules and cytokines in T lymphocytes by prolactin receptor knockdown.
Authors: Xu D, Lin L, Lin X
Cell. Immunol., 2010-03-02;263(1):71-8.
Species: Human
Sample Types: Cell Culture Supernates
-
IL-8 dictates glycosaminoglycan binding and stability of IL-18 in cystic fibrosis.
Authors: Reeves EP, Williamson M, Byrne B, Bergin DA, Smith SG, Greally P, O'Kennedy R, O'Neill SJ, McElvaney NG
J. Immunol., 2009-12-21;184(3):1642-52.
Species: Human
Sample Types: Serum
-
Fungal proteases induce Th2 polarization through limited dendritic cell maturation and reduced production of IL-12.
Authors: Lamhamedi-Cherradi SE, Martin RE, Ito T, Kheradmand F, Corry DB, Liu YJ, Moyle M
J. Immunol., 2008-05-01;180(9):6000-9.
Species: Human
Sample Types: Cell Culture Supernates
-
Human epithelial cells establish direct antifungal defense through TLR4-mediated signaling.
Authors: Weindl G, Naglik JR, Kaesler S, Biedermann T, Hube B, Korting HC, Schaller M
J. Clin. Invest., 2007-12-01;117(12):3664-3672.
Species: Human
Sample Types: Cell Culture Supernates
-
Calcium dependence of T cell proliferation following focal stimulation.
Authors: Schwarz EC, Kummerow C, Wenning AS, Wagner K, Sappok A, Waggershauser K, Griesemer D, Strauss B, Wolfs MJ, Quintana A, Hoth M
Eur. J. Immunol., 2007-10-01;37(10):2723-33.
Species: Human
Sample Types: Cell Culture Supernates
-
Immunotherapy for neuroblastoma using syngeneic fibroblasts transfected with IL-2 and IL-12.
Authors: Barker SE, Grosse SM, Siapati EK, Kritz A, Kinnon C, Thrasher AJ, Hart SL
Br. J. Cancer, 2007-06-26;97(2):210-7.
Species: Human
Sample Types: Cell Culture Supernates
-
Familial NK cell deficiency associated with impaired IL-2- and IL-15-dependent survival of lymphocytes.
Authors: Eidenschenk C, Jouanguy E, Alcais A, Mention JJ, Pasquier B, Fleckenstein IM, Puel A, Gineau L, Carel JC, Vivier E, Le Deist F, Casanova JL
J. Immunol., 2006-12-15;177(12):8835-43.
Species: Human
Sample Types: Cell Culture Supernates
-
Splice variants of human FOXP3 are functional inhibitors of human CD4+ T-cell activation.
Authors: Smith EL, Finney HM, Nesbitt AM, Ramsdell F, Robinson MK
Immunology, 2006-10-01;119(2):203-11.
Species: Human
Sample Types: Cell Culture Supernates
-
The Wingless homolog WNT5A and its receptor Frizzled-5 regulate inflammatory responses of human mononuclear cells induced by microbial stimulation.
Authors: Blumenthal A, Ehlers S, Lauber J, Buer J, Lange C, Goldmann T, Heine H, Brandt E, Reiling N
Blood, 2006-04-06;108(3):965-73.
Species: Human
Sample Types: Cell Culture Supernates
-
Human T cells constitutively express IL-15 that promotes ex vivo T cell homeostatic proliferation through autocrine/juxtacrine loops.
Authors: Miranda-Carus ME, Benito-Miguel M, Llamas MA, Balsa A, Martin-Mola E
J. Immunol., 2005-09-15;175(6):3656-62.
Species: Human
Sample Types: Cell Culture Supernates
-
Manipulation of host cytokine network by ticks: a potential gateway for pathogen transmission.
Authors: Hajnicka V, Vancova I, Kocakova P, Slovak M, Gasperik J, Slavikova M, Hails RS, Labuda M, Nuttall PA
Parasitology, 2005-03-01;130(0):333-42.
Species: Human
Sample Types: Saliva
-
Immune responses in normal Indian langur monkeys (Presbytis entellus)--a primate model for visceral leishmaniasis.
Authors: Misra A, Dube A, Naik S
J. Med. Primatol., 2004-04-01;33(2):65-9.
Species: Primate - Presbytis entellus (Indian langur)
Sample Types: Cell Culture Supernates
-
Activation of resting human primary T cells with chimeric receptors: costimulation from CD28, inducible costimulator, CD134, and CD137 in series with signals from the TCR zeta chain.
Authors: Finney HM, Akbar AN, Lawson AD
J. Immunol., 2004-01-01;172(1):104-13.
Species: Human
Sample Types: Cell Culture Supernates
-
Mycophenolic acid inhibits IL-2-dependent T cell proliferation, but not IL-2-dependent survival and sensitization to apoptosis.
Authors: Quemeneur L, Flacher M, Gerland LM, Ffrench M, Revillard JP, Bonnefoy-Berard N
J. Immunol., 2002-09-01;169(5):2747-55.
Species: Human
Sample Types: Cell Culture Supernates
FAQs
-
Is PBS a suitable alternative with which to formulate the Reagent Diluent?
Our lab has determined that Tris-buffered saline generates optimal assay performance in comparison to PBS for this assay. This assay is validated with Reagent Diluent formulated using Tris-buffered saline.
Reviews for Human IL-2 DuoSet ELISA
Average Rating: 4.5 (Based on 12 Reviews)
Have you used Human IL-2 DuoSet ELISA?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by: