Human ICAM-1/CD54 Antibody Summary
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
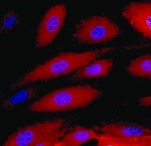
ICAM‑1/CD54 in C32 Human Cell Line. ICAM‑1/CD54 was detected in live unfixed C32 human melanoma cell line using Mouse Anti-Human ICAM‑1/CD54 Monoclonal Antibody (Catalog # BBA3) at 5 µg/mL for 1 hour at 2° - 8° C. Cells were then fixed with 4% formaldehyde followed by incubation with the NorthernLights™ 557-conjugated Anti-Mouse IgG Secondary Antibody (red; NL007) and counterstained with DAPI (blue). Specific staining was localized to cell surface and cytoplasm.
 View Larger
View Larger
Detection of ICAM‑1/CD54 in Human PBMCs by Flow Cytometry. Human peripheral blood mononuclear cells (PBMCs) were stained with (A) Mouse Anti-Human ICAM-1/CD54 Monoclonal Antibody (Catalog # BBA3) or (B) Mouse IgG1 Isotype Control (MAB002) followed by anti-Mouse IgG PE-conjugated Secondary Antibody (F0102B) and Mouse Anti-Human CD14 APC-conjugated Monoclonal Antibody (FAB3832A). Staining was performed using our Staining Membrane-associated Proteins protocol.
 View Larger
View Larger
Detection of Human ICAM‑1/CD54 by Western Blot. Western blot shows lysates of C32. PVDF membrane was probed with 2 µg/mL of Mouse Anti-Human ICAM‑1/CD54 Monoclonal Antibody (Catalog # BBA3) followed by HRP-conjugated Anti-Mouse IgG Secondary Antibody (Catalog # HAF018). A specific band was detected for ICAM‑1/CD54 at approximately 85 kDa (as indicated). This experiment was conducted under non-reducing conditions and using Western Blot Buffer Group 1.
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: ICAM-1/CD54
ICAM-1 is a member of the immunoglobulin superfamily whose expression is upregulated on leukocytes, epithelial cells and resting endothelial cells in response to inflammatory signals. ICAM-1 binds the leukocyte integrins LFA-1 and Mac-1.
Product Datasheets
Citations for Human ICAM-1/CD54 Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
81
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Modeling lung endothelial dysfunction in sepsis-associated ARDS using a microphysiological system
Authors: Liang, NW;Wilson, C;Davis, B;Wolf, I;Qyli, T;Moy, J;Beebe, DJ;Schnapp, LM;Kerr, SC;Faust, HE;
Physiological reports
Species: Human
Sample Types: Whole Cells
Applications: Immunocytochemistry -
Paracrinal regulation of neutrophil functions by coronaviral infection in iPSC-derived alveolar type II epithelial cells
Authors: Chien, Y;Huang, XY;Yarmishyn, AA;Chien, CS;Liu, YH;Hsiao, YJ;Lin, YY;Lai, WY;Huang, SC;Lee, MS;Chiou, SH;Yang, YP;Chiou, GY;
Virus research
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
ICAM-1 nanoclusters regulate hepatic epithelial cell polarity by leukocyte adhesion-independent control of apical actomyosin
Authors: Cacho-Navas, C;López-Pujante, C;Reglero-Real, N;Colás-Algora, N;Cuervo, A;Conesa, JJ;Barroso, S;de Rivas, G;Ciordia, S;Paradela, A;D'Agostino, G;Manzo, C;Feito, J;Andrés, G;Molina-Jiménez, F;Majano, P;Correas, I;Carazo, JM;Nourshargh, S;Huch, M;Millán, J;
eLife
Species: Human
Sample Types: Cell Lysates, Whole Cells
Applications: Immunoprecipitation, Immunocytochemistry -
Identification of new multi-substituted 1H-pyrazolo[3,4-c]pyridin-7(6H)-ones as soluble guanylyl cyclase (sGC) stimulators with vasoprotective and anti-inflammatory activities
Authors: Kintos, DP;Salagiannis, K;Sgouros, A;Nikolaropoulos, SS;Topouzis, S;Fousteris, MA;
Bioorganic chemistry
Species: Rat
Sample Types: Whole Cells
Applications: ELISA Capture -
Differential effects of Usutu and West Nile viruses on neuroinflammation, immune cell recruitment and blood–brain barrier integrity
Authors: Orianne Constant, Ghizlane Maarifi, Jonathan Barthelemy, Marie-France Martin, Bachirou Tinto, Giovanni Savini et al.
Emerging Microbes & Infections
-
Probing cerebral malaria inflammation in 3D human brain microvessels
Authors: Howard, C;Joof, F;Hu, R;Smith, JD;Zheng, Y;
Cell reports
Species: Human
Sample Types: Whole Cells
Applications: ICC -
The tumor stroma influences immune cell distribution and recruitment in a PDAC-on-a-chip model
Authors: Marlene Geyer, Lisa-Marie Gaul, Sabrina Luigia D`Agosto, Vincenzo Corbo, Karla Queiroz
Frontiers in Immunology
-
Vascular inflammation on a chip: A scalable platform for trans-endothelial electrical resistance and immune cell migration
Authors: Haley Ehlers, Arnaud Nicolas, Frederik Schavemaker, Jeroen P. M. Heijmans, Martin Bulst, Sebastiaan J. Trietsch et al.
Frontiers in Immunology
-
IL-33 Induces an Antiviral Signature in Mast Cells but Enhances Their Permissiveness for Human Rhinovirus Infection
Authors: C Akoto, A Willis, CF Banas, JA Bell, D Bryant, C Blume, DE Davies, EJ Swindle
Viruses, 2022-11-01;14(11):.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
The impact of macrophages on endothelial cells is potentiated by cycling hypoxia: Enhanced tumor inflammation and metastasis
Authors: Victor Delprat, Camille Huart, Olivier Feron, Fabrice Soncin, Carine Michiels
Frontiers in Oncology
-
A microengineered Brain-Chip to model neuroinflammation in humans
Authors: Iosif Pediaditakis, Konstantia R. Kodella, Dimitris V. Manatakis, Christopher Y. Le, Sonalee Barthakur, Alexander Sorets et al.
iScience
-
GAS6/TAM signaling pathway controls MICA expression in multiple myeloma cells
Authors: Andrea Kosta, Abdelilah Mekhloufi, Lorenzo Lucantonio, Alessandra Zingoni, Alessandra Soriani, Marco Cippitelli et al.
Frontiers in Immunology
-
IFNgamma-primed periodontal ligament cells regulate T-cell responses via IFNgamma-inducible mediators and ICAM-1-mediated direct cell contact
Authors: W Singhatana, S Kitpakorns, M Toso, P Pavasant
Royal Society open science, 2022-07-27;9(7):220056.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Plasmolipin regulates basolateral-to-apical transcytosis of ICAM-1 and leukocyte adhesion in polarized hepatic epithelial cells
Authors: Cristina Cacho-Navas, Natalia Reglero-Real, Natalia Colás-Algora, Susana Barroso, Gema de Rivas, Kostantinos Stamatakis et al.
Cellular and Molecular Life Sciences
-
Endothelial cells are not productively infected by SARS‐CoV‐2
Authors: Lilian Schimmel, Keng Yih Chew, Claudia J Stocks, Teodor E Yordanov, Patricia Essebier, Arutha Kulasinghe et al.
Clinical & Translational Immunology
-
Shear forces induce ICAM-1 nanoclustering on endothelial cells that impact on T-cell migration
Authors: Izabela K. Piechocka, Sarah Keary, Alberto Sosa-Costa, Lukas Lau, Nitin Mohan, Jelena Stanisavljevic et al.
Biophysical Journal
-
Plasmacytoid dendritic cells have divergent effects on HIV infection of initial target cells and induce a pro-retention phenotype
Authors: O Tong, G Duette, TR O'Neil, CM Royle, H Rana, B Johnson, N Popovic, S Dervish, MAE Brouwer, H Baharlou, E Patrick, G Ctercteko, S Palmer, E Lee, E Hunter, AN Harman, AL Cunningham, N Nasr
PloS Pathogens, 2021-04-19;17(4):e1009522.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
CD112 Regulates Angiogenesis and T Cell Entry into the Spleen
Authors: E Russo, P Runge, NH Jahromi, H Naboth, A Landtwing, R Montecchi, N Leicht, MC Hunter, Y Takai, C Halin
Cells, 2021-01-15;10(1):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Methotrexate attenuates vascular inflammation through an adenosine-microRNA-dependent pathway
Authors: Dafeng Yang, Stefan Haemmig, Haoyang Zhou, Daniel Pérez-Cremades, Xinghui Sun, Lei Chen et al.
eLife
-
Human CD4+ T cell subsets differ in their abilities to cross endothelial and epithelial brain barriers in vitro
Authors: Hideaki Nishihara, Sasha Soldati, Adrien Mossu, Maria Rosito, Henriette Rudolph, William A. Muller et al.
Fluids and Barriers of the CNS
-
Neutrophils interact with cholangiocytes to cause cholestatic changes in alcoholic hepatitis
Authors: Masahiro Takeuchi, Paula T Vidigal, Mateus T Guerra, Melanie A Hundt, Marie E Robert, Maria Olave-Martinez et al.
Gut
-
Caspase-1 has a critical role in blood-brain barrier injury and its inhibition contributes to multifaceted repair
Authors: H Israelov, O Ravid, D Atrakchi, D Rand, S Elhaik, Y Bresler, R Twitto-Gre, L Omesi, S Liraz-Zalt, F Gosselet, M Schnaider, I Cooper
J Neuroinflammation, 2020-09-09;17(1):267.
Species: Human
Sample Types: Whole Cells
Applications: ICC, Western Blot -
Zika Virus Infection Promotes Local Inflammation, Cell Adhesion Molecule Upregulation, and Leukocyte Recruitment at the Blood-Brain Barrier
Authors: M Clé, C Desmetz, J Barthelemy, MF Martin, O Constant, G Maarifi, V Foulongne, K Bolloré, Y Glasson, F De Bock, M Blaquiere, L Dehouck, N Pirot, E Tuaillon, S Nisole, F Najioullah, P Van de Per, A Cabié, N Marchi, F Gosselet, Y Simonin, S Salinas
MBio, 2020-08-04;11(4):.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
hnRNPA2/B1 Ameliorates LPS-Induced Endothelial Injury through NF-&kappaB Pathway and VE-Cadherin/&beta-Catenin Signaling Modulation In Vitro
Authors: Y Chen, D Tang, L Zhu, T Yuan, Y Jiao, H Yan, W Yu
Mediators Inflamm., 2020-05-30;2020(0):6458791.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Hepatocytes Delete Regulatory T Cells by Enclysis, a CD4+ T Cell Engulfment Process
Authors: SP Davies, GM Reynolds, AL Wilkinson, X Li, R Rose, M Leekha, YS Liu, R Gandhi, E Buckroyd, J Grove, NM Barnes, RC May, SG Hubscher, DH Adams, Y Huang, O Qureshi, Z Stamataki
Cell Rep, 2019-11-05;29(6):1610-1620.e4.
Species: Human
Sample Types: Whole Cells
Applications: IF -
Dual complementary liposomes inhibit triple-negative breast tumor progression and metastasis
Authors: P Guo, J Yang, D Liu, L Huang, G Fell, J Huang, MA Moses, DT Auguste
Sci Adv, 2019-03-20;5(3):eaav5010.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
3D human microvessel-on-a-chip model for studying monocyte-to-endothelium adhesion under flow – application in systems toxicology
Authors: Carine Poussin, Bart Kramer, Henriette L Lanz, Angelique Van den Heuvel, Alexandra Laurent, Thomas Olivier et al.
ALTEX
-
Hypoxia and matrix viscoelasticity sequentially regulate endothelial progenitor cluster-based vasculogenesis
Authors: MR Blatchley, F Hall, S Wang, HC Pruitt, S Gerecht
Sci Adv, 2019;5(3):eaau7518.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
Blood outgrowth endothelial cells (BOECs) as a novel tool for studying adhesion of Plasmodium falciparum-infected erythrocytes
Authors: G Ecklu-Mens, RW Olsen, A Bengtsson, MF Ofori, L Hviid, ATR Jensen, Y Adams
PLoS ONE, 2018-10-09;13(10):e0204177.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry, ICC -
Immune complexes containing scleroderma-specific autoantibodies induce a profibrotic and proinflammatory phenotype in skin fibroblasts
Authors: E Raschi, CB Chighizola, L Cesana, D Privitera, F Ingegnoli, C Mastaglio, PL Meroni, MO Borghi
Arthritis Res. Ther., 2018-08-29;20(1):187.
Species: Human
Sample Types: Whole Cells
Applications: Inhibition -
Protective effect of KLF15 on vascular endothelial dysfunction induced by TNF??.
Authors: Bing Liu, Lili Xu, Xinming Yu, Wei Li, Xiaozhi Sun, Shun Xiao, Mingjin Guo, Haofu Wang
Molecular Medicine Reports, 2018-06-20;0(0):1791-3004.
Species: Human
Sample Types: Cell Lysate, Cell Lysates
Applications: Western Blot -
Pinocembrin protects endothelial cells from oxidized LDL-induced injury
Authors: Q Su, Y Sun, Z Ye, H Yang, B Kong, L Li
Cytokine, 2018-06-18;0(0):.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Augmented reduction in colonic inflammatory markers of dextran sulfate sodium-induced colitis with a combination of 5-aminosalicylic acid and AD-lico™ from Glycyrrhiza inflata
Authors: Jaeyoung Cho, Hyuck-Se Kweon, Sung-Oh Huh, Ali Sadra
Animal Cells and Systems
-
Matrix metalloproteinase-9 (MMP9) is involved in the TNF-α-induced fusion of human M13SV1-Cre breast epithelial cells and human MDA-MB-435-pFDR1 cancer cells
Authors: J Weiler, M Mohr, KS Zänker, T Dittmar
Cell Commun. Signal, 2018-04-10;16(1):14.
Species: Human
Sample Types: Cell Lysates, Whole Cells
Applications: Immunoprecipitation, Neutralization -
Inflammation-Sensitive Myosin-X Functionally Supports Leukocyte Extravasation by Cdc42-Mediated ICAM-1-Rich Endothelial Filopodia Formation
Authors: J Kroon, A Schaefer, J van Rijsse, M Hoogenboez, F van Alphen, P Hordijk, ESG Stroes, S Strömblad, J van Rheene, JD van Buul
J. Immunol., 2018-01-31;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bead-based Bioassay, ICC -
DL-3-n-butylphthalide protects endothelial cells against advanced glycation end product-induced injury by attenuating oxidative stress and inflammation responses
Authors: Chang-Yun Liu, Zhen-Hua Zhao, Zhi-Ting Chen, Chun-Hui Che, Zhang-Yu Zou, Xiao-Min Wu et al.
Experimental and Therapeutic Medicine
-
ALCAM (CD166) is involved in extravasation of monocytes rather than T cells across the blood–brain barrier
Authors: Ruth Lyck, Marc-André Lécuyer, Michael Abadier, Christof B Wyss, Christoph Matti, Maria Rosito et al.
Journal of Cerebral Blood Flow & Metabolism
-
Endothelial CD2AP Binds the Receptor ICAM-1 To Control Mechanosignaling, Leukocyte Adhesion, and the Route of Leukocyte Diapedesis In Vitro
Authors: A Schaefer, TJ van Duijn, J Majolee, K Burridge, PL Hordijk
J. Immunol., 2017-05-08;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Functional Assay, ICC -
CD54-Mediated Interaction with Pro-inflammatory Macrophages Increases the Immunosuppressive Function of Human Mesenchymal Stromal Cells
Authors: Nicolas Espagnolle, Adélie Balguerie, Emmanuelle Arnaud, Luc Sensebé, Audrey Varin
Stem Cell Reports
-
A quantitative method for screening and identifying molecular targets for nanomedicine
Authors: P Guo, J Yang, DR Bielenberg, D Dillon, D Zurakowski, MA Moses, DT Auguste
J Control Release, 2017-03-22;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
A possible role for neutrophils in allergic rhinitis revealed after cellular subclassification
Authors: J Arebro, S Ekstedt, E Hjalmarsso, O Winqvist, S Kumlien Ge, LO Cardell
Sci Rep, 2017-03-08;7(0):43568.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
3D co-culture model to analyze the crosstalk between endothelial and smooth muscle cells
Authors: Minu Karthika Ganesan
Tissue Eng Part C Methods, 2017-01-01;0(0):.
Species: Human
Sample Types: Complex Sample Type
Applications: ICC -
A novel immune resistance mechanism of melanoma cells controlled by the ADAR1 enzyme
Authors: Gilli Galore-Haskel, Yael Nemlich, Eyal Greenberg, Shira Ashkenazi, Motti Hakim, Orit Itzhaki et al.
Oncotarget
-
C6-ceramide nanoliposome suppresses tumor metastasis by eliciting PI3K and PKCzeta tumor-suppressive activities and regulating integrin affinity modulation.
Authors: Zhang P, Fu C, Hu Y, Dong C, Song Y, Song E
Sci Rep, 2015-03-20;5(0):9275.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Role of cortactin homolog HS1 in transendothelial migration of natural killer cells.
Authors: Mukherjee S, Kim J, Mooren O, Shahan S, Cohan M, Cooper J
PLoS ONE, 2015-02-27;10(2):e0118153.
Species: Human
Sample Types: Whole Cells
Applications: IHC -
Cycling Hypoxia Induces a Specific Amplified Inflammatory Phenotype in Endothelial Cells and Enhances Tumor-Promoting Inflammation In Vivo12
Authors: Céline Tellier, Déborah Desmet, Laurenne Petit, Laure Finet, Carlos Graux, Martine Raes et al.
Neoplasia
-
Soluble CD54 induces human endothelial cells ex vivo expansion useful for cardiovascular regeneration and tissue engineering application.
Authors: Malara N, Trunzo V, Musolino G, Aprigliano S, Rotta G, Macrina L, Limongi T, Gratteri S, Di Fabrizio E, Renzulli A, Fini M, Mollace V
Int J Cardiol Heart Vasc, 2015-01-09;6(0):48-53.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Uveal melanoma cells utilize a novel route for transendothelial migration.
Authors: Onken, Michael, Li, Jinmei, Cooper, John A
PLoS ONE, 2014-12-15;9(12):e115472.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Endothelial cells use dynamic actin to facilitate lymphocyte transendothelial migration and maintain the monolayer barrier.
Authors: Mooren O, Li J, Nawas J, Cooper J
Mol Biol Cell, 2014-10-29;25(25):4115-29.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
West Nile virus-induced cell adhesion molecules on human brain microvascular endothelial cells regulate leukocyte adhesion and modulate permeability of the in vitro blood-brain barrier model.
Authors: Roe, Kelsey, Orillo, Beverly, Verma, Saguna
PLoS ONE, 2014-07-18;9(7):e102598.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Phenolic acid composition, antiatherogenic and anticancer potential of honeys derived from various regions in Greece.
Authors: Spilioti E, Jaakkola M, Tolonen T, Lipponen M, Virtanen V, Chinou I, Kassi E, Karabournioti S, Moutsatsou P
PLoS ONE, 2014-04-21;9(4):e94860.
Species: Human
Sample Types: Whole Cells
Applications: ELISA Development (Capture) -
Inflammation converts human mesoangioblasts into targets of alloreactive immune responses: implications for allogeneic cell therapy of DMD.
Authors: Noviello M, Tedesco F, Bondanza A, Tonlorenzi R, Rosaria Carbone M, Gerli M, Marktel S, Napolitano S, Cicalese M, Ciceri F, Peretti G, Cossu G, Bonini C
Mol Ther, 2014-04-16;22(7):1342-52.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Enzyme-free passage of human pluripotent stem cells by controlling divalent cations.
Authors: Ohnuma, Kiyoshi, Fujiki, Ayaka, Yanagihara, Kana, Tachikawa, Saoko, Hayashi, Yohei, Ito, Yuzuru, Onuma, Yasuko, Chan, Techuan, Michiue, Tatsuo, Furue, Miho K, Asashima, Makoto
Sci Rep, 2014-04-11;4(0):4646.
Species: Human
Sample Types: Whole Cells
Applications: IHC -
Interaction of mesenchymal stem cells with fibroblast-like synoviocytes via cadherin-11 promotes angiogenesis by enhanced secretion of placental growth factor.
Authors: Park, Su-Jung, Kim, Ki-Jo, Kim, Wan-Uk, Cho, Chul-Soo
J Immunol, 2014-02-26;192(7):3003-10.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
GroEL1, a heat shock protein 60 of Chlamydia pneumoniae, impairs neovascularization by decreasing endothelial progenitor cell function.
Authors: Lin, Yi-Wen, Huang, Chun-Yao, Chen, Yung-Hsi, Shih, Chun-Min, Tsao, Nai-Wen, Lin, Cheng-Ye, Chang, Nen-Chun, Tsai, Chien-Su, Tsai, Hsiao-Ya, Tsai, Jui-Chi, Huang, Po-Hsun, Li, Chi-Yuan, Lin, Feng-Yen
PLoS ONE, 2013-12-23;8(12):e84731.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
A composite model of the human postcapillary venule for investigation of microvascular leukocyte recruitment.
Authors: Lauridsen H, Pober J, Gonzalez A
FASEB J, 2013-12-02;28(3):1166-80.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Zoledronate attenuates angiotensin II-induced abdominal aortic aneurysm through inactivation of Rho/ROCK-dependent JNK and NF-kappaB pathway.
Authors: Tsai S, Huang P, Peng Y, Chang W, Tsai H, Leu H, Chen J, Lin S
Cardiovasc Res, 2013-12-01;100(3):501-10.
Species: Mouse
Sample Types: Tissue Homogenates
Applications: Western Blot -
Tumor-associated fibroblasts isolated from colorectal cancer tissues exhibit increased ICAM-1 expression and affinity for monocytes.
Authors: Schellerer V, Langheinrich M, Hohenberger W, Croner R, Merkel S, Rau T, Sturzl M, Naschberger E
Oncol Rep, 2013-11-20;31(1):255-61.
Species: Human
Sample Types: Whole Cells, Whole Tissue
Applications: ICC, IHC-Fr -
Loss of p53 in stromal fibroblasts promotes epithelial cell invasion through redox-mediated ICAM1 signal
Authors: Dunyaporn Trachootham, Gang Chen, Wan Zhang, Weiqin Lu, Hui Zhang, Jinsong Liu et al.
Free Radical Biology and Medicine
-
Macrophages induce differentiation of plasma cells through CXCL10/IP-10.
J. Exp. Med., 2012-09-17;209(10):1813-23.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Activation of endothelial TLR2 by bacterial lipoprotein upregulates proteins specific for the neutrophil response
Authors: Kevin Wilhelmsen, Kailin R Mesa, Arun Prakash, Fengyun Xu, Judith Hellman
Innate Immunity
-
OxLDL and substrate stiffness promote neutrophil transmigration by enhanced endothelial cell contractility and ICAM-1
Authors: Kimberly M. Stroka, Irena Levitan, Helim Aranda-Espinoza
Journal of Biomechanics
-
ERK5 protein promotes, whereas MEK1 protein differentially regulates, the Toll-like receptor 2 protein-dependent activation of human endothelial cells and monocytes.
Authors: Wilhelmsen K, Mesa K, Lucero J, Xu F, Hellman J
J Biol Chem, 2012-06-15;287(32):26478-94.
Species: Human
Sample Types: Whole Cells
Applications: Cell-based ELISA -
Endothelial cell substrate stiffness influences neutrophil transmigration via myosin light chain kinase-dependent cell contraction.
Authors: Stroka KM, Aranda-Espinoza H
Blood, 2011-06-07;118(6):1632-40.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Deficiency of the NR4A Orphan Nuclear Receptor NOR1 Decreases Monocyte Adhesion and Atherosclerosis
Authors: Yue Zhao, Deborah A. Howatt, Florence Gizard, Takashi Nomiyama, Hannes M. Findeisen, Elizabeth B. Heywood et al.
Circulation Research
-
IL-17 amplifies human contact hypersensitivity by licensing hapten nonspecific Th1 cells to kill autologous keratinocytes.
Authors: Pennino D, Eyerich K, Scarponi C, Carbone T, Eyerich S, Nasorri F, Garcovich S, Traidl-Hoffmann C, Albanesi C, Cavani A
J. Immunol., 2010-03-31;184(9):4880-8.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Adhesion of human haematopoietic (CD34+) stem cells to human liver compartments is integrin and CD44 dependent and modulated by CXCR3 and CXCR4.
Authors: Crosby HA, Lalor PF, Ross E, Newsome PN, Adams DH
J. Hepatol., 2009-07-30;51(4):734-49.
Species: Human
Sample Types: Whole Tissue
Applications: IHC, Neutralization -
Cannabidiol attenuates high glucose-induced endothelial cell inflammatory response and barrier disruption.
Authors: Rajesh M, Mukhopadhyay P, Batkai S, Hasko G, Liaudet L, Drel VR, Obrosova IG, Pacher P
Am. J. Physiol. Heart Circ. Physiol., 2007-03-23;293(1):H610-9.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Glucocorticoid-induced tumour necrosis factor receptor-related protein-mediated macrophage stimulation may induce cellular adhesion and cytokine expression in rheumatoid arthritis.
Authors: Bae E, Kim WJ, Kang YM, Suk K, Koh EM, Cha HS, Ahn KS, Huh TL, Lee WH
Clin. Exp. Immunol., 2007-03-15;148(3):410-8.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
SPARC is a VCAM-1 counter-ligand that mediates leukocyte transmigration.
Authors: Kelly KA, Allport JR, Yu AM, Sinh S, Sage EH, Gerszten RE, Weissleder R
J. Leukoc. Biol., 2006-12-18;81(3):748-56.
Species: Human
Sample Types: Whole Cells
Applications: Functional Assay -
Differential survival of leukocyte subsets mediated by synovial, bone marrow, and skin fibroblasts: site-specific versus activation-dependent survival of T cells and neutrophils.
Authors: Filer A, Parsonage G, Smith E, Osborne C, Thomas AM, Curnow SJ, Rainger GE, Raza K, Nash GB, Lord J, Salmon M, Buckley CD
Arthritis Rheum., 2006-07-01;54(7):2096-108.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Proteinase-activated receptor2 agonists upregulate granulocyte colony-stimulating factor, IL-8, and VCAM-1 expression in human bronchial fibroblasts.
Authors: Ramachandran R, Morice AH, Compton SJ
Am. J. Respir. Cell Mol. Biol., 2006-02-23;35(1):133-41.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Migration inhibitory factor up-regulates vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 via Src, PI3 kinase, and NFkappaB.
Authors: Amin MA, Haas CS, Zhu K, Mansfield PJ, Kim MJ, Lackowski NP, Koch AE
Blood, 2005-11-29;107(6):2252-61.
Species: Human
Sample Types: Cell Lysates, Whole Cells
Applications: ICC, Western Blot -
Cell expression of MMP-1 and TIMP-1 in co-cultures of human gingival fibroblasts and monocytes: the involvement of ICAM-1.
Authors: Domeij H, Modeer T, Quezada HC, Yucel-Lindberg T
Biochem. Biophys. Res. Commun., 2005-11-02;338(4):1825-33.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Requirement for intercellular adhesion molecule 1 and caveolae in invasion of human oral epithelial cells by Porphyromonas gingivalis.
Authors: Tamai R, Asai Y, Ogawa T
Infect. Immun., 2005-10-01;73(10):6290-8.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Differential effects of orbital and laminar shear stress on endothelial cells.
Authors: Frattini J, Kudo FA
J. Vasc. Surg., 2005-05-01;41(5):869-80.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Persistent cytomegalovirus infection is associated with increased expression of TGF-beta1, PDGF-AA and ICAM-1 and arterial intimal thickening in kidney allografts.
Authors: Helantera I, Loginov R, Koskinen P, Tornroth T, Gronhagen-Riska C, Lautenschlager I
Nephrol. Dial. Transplant., 2005-02-16;20(4):790-6.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
CC chemokines and transmigration of eosinophils in the presence of vascular cell adhesion molecule 1.
Authors: Yamamoto H, Nagata M, Sakamoto Y
Ann. Allergy Asthma Immunol., 2005-02-01;94(2):292-300.
Species: Human
Sample Types: Cell Culture Supernates
Applications: ELISA Development -
Apical topography and modulation of ICAM-1 expression on activated endothelium.
Authors: Almenar-Queralt A, Duperray A, Miles LA, Felez J, Altieri DC
Am. J. Pathol., 1995-11-01;147(5):1278-88.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry, ICC -
Circulating tumor cells from prostate cancer patients interact with E-selectin under physiologic blood flow.
Authors: Gakhar, Gunjan, Navarro, Vicente, Jurish, Madelyn, Lee, Guang Yu, Tagawa, Scott T, Akhtar, Naveed H, Seandel, Marco, Geng, Yue, Liu, He, Bander, Neil H, Giannakakou, Paraskev, Christos, Paul J, King, Michael, Nanus, David M
PLoS ONE, 2013-12-27;8(12):e85143.
-
LncRNA VINAS regulates atherosclerosis by modulating NF-&kappaB and MAPK signaling
Authors: V Simion, H Zhou, JB Pierce, D Yang, S Haemmig, Y Tesmenitsk, G Sukhova, PH Stone, P Libby, MW Feinberg
JCI Insight, 2020-11-05;0(0):.
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Human ICAM-1/CD54 Antibody
Average Rating: 5 (Based on 2 Reviews)
Have you used Human ICAM-1/CD54 Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by: