Human CD44s Pan Specific Antibody Summary
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
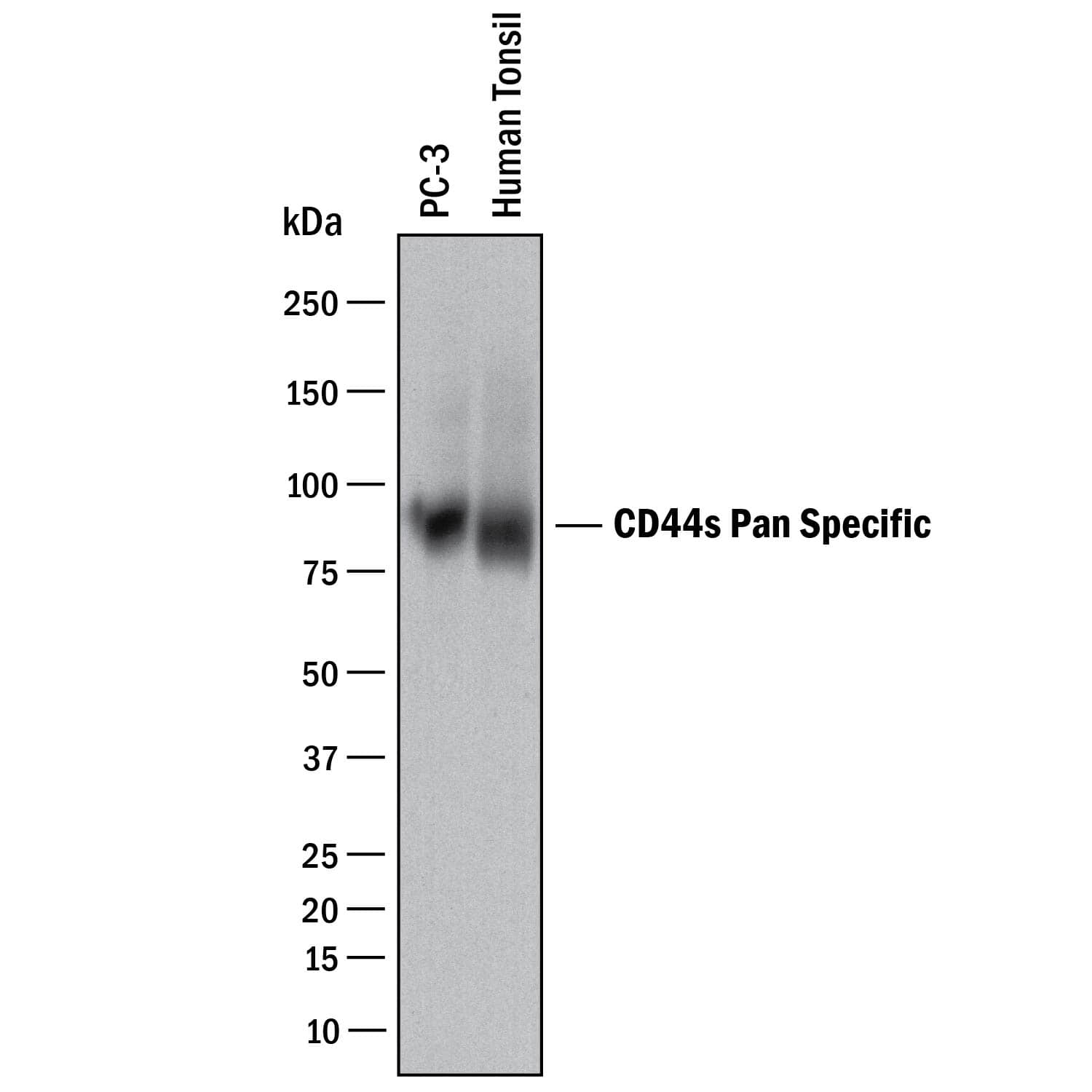
Detection of Human CD44 by Western Blot. Western blot shows lysates of PC-3 human prostate cancer cell line and human tonsil tissue. PVDF membrane was probed with 2 µg/mL of Mouse Anti-Human CD44 s Pan Specific Monoclonal Antibody (Catalog # BBA10) followed by HRP-conjugated Anti-Mouse IgG Secondary Antibody (HAF018). A specific band was detected for CD44 at approximately 90 kDa (as indicated). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 1.
 View Larger
View Larger
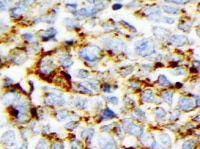
CD44 in Human Lymphoma. CD44 was detected in immersion fixed paraffin-embedded sections of human lymphoma using Mouse Anti-Human CD44 s Pan Specific Monoclonal Antibody (Catalog # BBA10) at 15 µg/mL overnight at 4 °C. Tissue was stained using the Anti-Mouse HRP-DAB Cell & Tissue Staining Kit (brown; CTS002) and counterstained with hematoxylin (blue). Specific staining was localized to plasma membrane. View our protocol for Chromogenic IHC Staining of Paraffin-embedded Tissue Sections.
 View Larger
View Larger
Detection of Human CD44 by Simple WesternTM. Simple Western lane view shows lysates of human tonsil tissue, loaded at 0.2 mg/mL. A specific band was detected for CD44 at approximately 156 kDa (as indicated) using 20 µg/mL of Mouse Anti-Human CD44 s Pan Specific Monoclonal Antibody (Catalog # BBA10). This experiment was conducted under reducing conditions and using the 12-230 kDa separation system.
 View Larger
View Larger
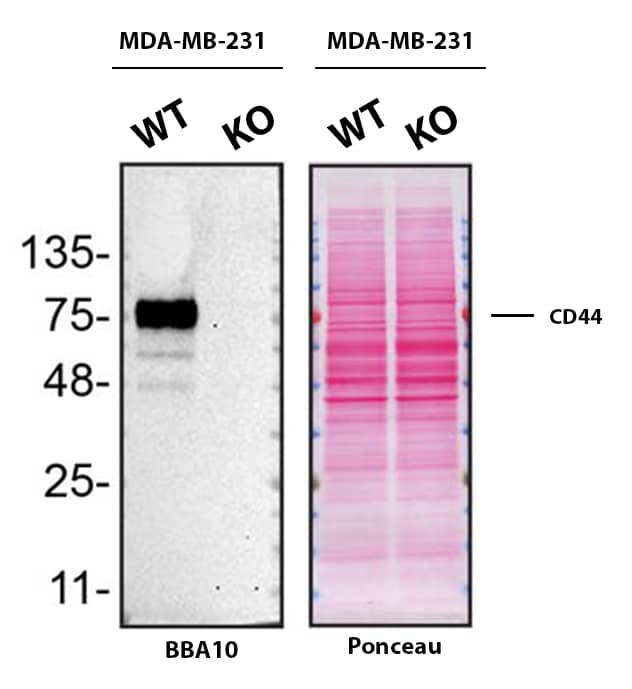
Western Blot Shows CD44 Specificity Using Knockout Cell Line. Western blot shows lysates of MDA‑MB‑231 human breast cancer parental cell line and CD44 knockout MDA-MB-231 cell line (KO). Nitrocellulose membrane was probed with Mouse Anti-Human CD44 s Pan Specific Monoclonal Antibody (Catalog # BBA10) followed by HRP-conjugated secondary antibody. A specific band was detected for CD44 at approximately 81.5 kDa (as indicated) in the parental MDA-MB-231 cell line, but is not detectable in knockout MDA-MB-231 cell line. Primary antibody concentration used: 2.5 µg/mL. The Ponceau stained transfer of the blot is shown. This experiment was conducted under reducing conditions. Image, protocol, and testing courtesy of YCharOS Inc. See ycharos.com for additional details.
 View Larger
View Larger
Detection of CD44 by Immunoprecipitation. MDA‑MB‑231 human breast cancer cell line lysates were prepared and immunoprecipitation was performed using 2.0 μg of Mouse Anti-Human CD44 s Pan Specific Monoclonal Antibody (Catalog # BBA10) pre-coupled to Dynabeads Protein G. Immunoprecipitated CD44 was detected in Western Blot with a rabbit CD44 antibody used at 1/3000. The Ponceau stained transfer of the blot is shown. SM=4% starting material; UB=4% unbound fraction; IP=immunoprecipitate; HC=antibody heavy chain. Image, protocol and testing courtesy of YCharOS Inc. (ycharos.com).
 View Larger
View Larger
CD44 Specificity is Shown by Immunocytochemistry in Knockout Cell Line. MDA‑MB‑231 human breast cancer parental cell line WT and CD44 MDA-MB-231 KO cells were labelled with a green or a far-red fluorescent dye, respectively. Cells were stained with Mouse Anti-Human CD44 s Pan Specific Monoclonal Antibody (Catalog # BBA10) followed by incubation with an Alexa-fluor 555 conjugated secondary antibody (upper panel). DAPI-only counterstained cells shown on a lower panel. Acquisition of the blue (nucleus-DAPI), green (identification of WT cells), red (antibody staining) and far-red (identification of KO cells) channels was performed. Representative images of the blue and red (grayscale) channels are shown. WT and KO cells are outlined with green and magenta dashed line, respectively. Primary antibody concentration used: 2 μg/mL. Image, protocol and testing courtesy of YCharOS Inc. (ycharos.com).
 View Larger
View Larger
Detection of CD44 by Flow Cytometry. MDA‑MB‑231 human breast cancer parental cell line WT and CD44 KO cells were labelled with a green or violet, fluorescent dye, respectively. WT and KO cells were mixed in a 1:1 ratio, fixed in 4% PFA and permeabilized in 0.1% saponin. 400,000 cells were stained with Mouse Anti-Human CD44 s Pan Specific Monoclonal Antibody (Catalog # BBA10) and a secondary antibody. Antibody staining was quantified, and representative images showing the staining intensity in the KO population (pink histogram, dashed line) compared to the WT cells (green histogram, solid line) are presented. Histograms with dotted lines represent secondary antibody-only controls in both WT and KO cells. Primary antibody concentration: 1 μg/mL. Image, protocol and testing courtesy of YCharOS Inc. (ycharos.com).
 View Larger
View Larger
Detection of Human CD44 by Immunocytochemistry/Immunofluorescence Differential localization of Kv1.3 and KCa3.1 in migrating T cells.A. Distribution of Kv1.3 and KCa3.1 at the uropod. T cells were transiently transfected with either YFP-KCa3.1 or GFP-Kv1.3 (green) and stained with anti-CD44 antibody (uropod; red) without permeabilization. Yellow areas in the merge images indicate colocalization. Scale bar = 5 µm. B. Distribution of Kv1.3 and KCa3.1 at the leading-edge. T cells, transfected with either YFP-KCa3.1 or GFP-Kv1.3 (green) and stained with anti-CXCR-4 antibody (leading-edge; red) without permeabilization, were analyzed by confocal microscopy. Colocalization between the two proteins is indicated by yellow areas in the merge images. Scale bar = 5 µm. C. Correlation coefficients for KCa3.1 and Kv1.3 localization in the uropod (U) and leading-edge (L). The data are the average of n = 15 cells for KCa3.1 at the U and n = 8 at the L, and n = 16 for Kv1.3 at the U and n = 11 at the U from 2 healthy individuals. Statistical significance was established by one way ANOVA. D. Localization of native Kv1.3 in the leading-edge. T cells from one healthy individual were fixed and stained with extracellular anti-Kv1.3 antibody (green) together with antibodies either against CD44 (red; left) or CXCR-4 (red, right). Yellow colors in the merge images indicate strong correlation. Scale bar = 5 µm. E. Average Correlation coefficients of native Kv1.3 with the leading-edge (L) (n = 9) and the uropod (U) markers (n = 11). Image collected and cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0043859), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human CD44 by Immunocytochemistry/Immunofluorescence Differential localization of Kv1.3 and KCa3.1 in migrating T cells.A. Distribution of Kv1.3 and KCa3.1 at the uropod. T cells were transiently transfected with either YFP-KCa3.1 or GFP-Kv1.3 (green) and stained with anti-CD44 antibody (uropod; red) without permeabilization. Yellow areas in the merge images indicate colocalization. Scale bar = 5 µm. B. Distribution of Kv1.3 and KCa3.1 at the leading-edge. T cells, transfected with either YFP-KCa3.1 or GFP-Kv1.3 (green) and stained with anti-CXCR-4 antibody (leading-edge; red) without permeabilization, were analyzed by confocal microscopy. Colocalization between the two proteins is indicated by yellow areas in the merge images. Scale bar = 5 µm. C. Correlation coefficients for KCa3.1 and Kv1.3 localization in the uropod (U) and leading-edge (L). The data are the average of n = 15 cells for KCa3.1 at the U and n = 8 at the L, and n = 16 for Kv1.3 at the U and n = 11 at the U from 2 healthy individuals. Statistical significance was established by one way ANOVA. D. Localization of native Kv1.3 in the leading-edge. T cells from one healthy individual were fixed and stained with extracellular anti-Kv1.3 antibody (green) together with antibodies either against CD44 (red; left) or CXCR-4 (red, right). Yellow colors in the merge images indicate strong correlation. Scale bar = 5 µm. E. Average Correlation coefficients of native Kv1.3 with the leading-edge (L) (n = 9) and the uropod (U) markers (n = 11). Image collected and cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0043859), licensed under a CC-BY license. Not internally tested by R&D Systems.
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: CD44
CD44 is a ubiquitously expressed protein that is the major receptor for hyaluronan and exerts control over cell growth and migration (1-3). Human CD44 has a 20 amino acid (aa) signal sequence, an extracellular domain (ECD) with a 100 aa hyaluronan-binding disulfide-stabilized link region and a 325-530 aa stem region, a 21 aa transmembrane domain, and a 72 aa cytoplasmic domain. Within the stem, ten variably spliced exons (v1-10, exons 6-15) produce multiple protein isoforms (1‑3). The standard or hematopoietic form, CD44s, does not include the variable segments (1‑3). Cancer aggressiveness and T cell activation have been correlated with expression of specific isoforms (1, 3). With variable N- and O-glycosylation and splicing within the stalk, CD44 can range from 80-200 kDa (1). Within the N‑terminal invariant portion of the ECD (aa 21-220), human CD44 shares 76%, 76%, 86%, 83%, and 79% identity with corresponding mouse, rat, equine, canine, and bovine CD44, respectively. The many reported functions of CD44 fall within three categories (1). First, CD44 binds hyaluronan and other ligands within the extracellular matrix and can function as a “platform” for growth factors and metalloproteinases. Second, CD44 can function as a co-receptor that modifies activity of receptors including MET and the ERBB family of tyrosine kinases. Third, the CD44 intracellular domain links the plasma membrane to the actin cytoskeleton via the ERM proteins, ezrin, radixin and moesin. CD44 can be synthesized in a soluble form (4) or may be cleaved at multiple sites by either membrane-type matrix metalloproteinases, or ADAM proteases to produce soluble ectodomains (5, 6). The cellular portion may then undergo gamma secretase-dependent intramembrane cleavage to form an A beta -like transmembrane portion and a cytoplasmic signaling portion that affects gene expression (7, 8). These cleavage events are thought to promote metastasis by enhancing tumor cell motility and growth (1, 5).
- Ponta, H. et al. (2003) Nat. Rev. Mol. Cell Biol. 4:33.
- Screaton, G.R. et al. (1992) Proc. Natl. Acad. Sci. USA 89:12160.
- Lynch, K.W. (2004) Nat. Rev. Immunol. 4:931.
- Yu, Q. and B.P. Toole (1996) J. Biol. Chem. 271:20603.
- Nagano, O. and H. Saya (2004) Cancer Sci. 95:930.
- Nakamura, H. et al. (2004) Cancer Res. 64:876.
- Murakami, D. et al. (2003) Oncogene 22:1511.
- Lammich, S. et al. (2002) J. Biol. Chem. 277:44754.
Product Datasheets
Citations for Human CD44s Pan Specific Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
49
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
FKBPL and its peptide derivatives inhibit endocrine therapy resistant cancer stem cells and breast cancer metastasis by downregulating DLL4 and Notch4
Authors: L McClements, S Annett, A Yakkundi, M O'Rourke, A Valentine, N Moustafa, A Alqudah, BM Simões, F Furlong, A Short, SA McIntosh, HO McCarthy, RB Clarke, T Robson
BMC Cancer, 2019-04-11;19(1):351.
-
Divergent single cell transcriptome and epigenome alterations in ALS and FTD patients with C9orf72 mutation
Authors: Li J, Jaiswal MK, Chien JF et al.
Nat Commun
Applications: Simple Western -
Enhancing Transcriptional Reprogramming of Mesenchymal Glioblastoma with Grainyhead-like 2 and HDAC Inhibitors Leads to Apoptosis and Cell-Cycle Dysregulation
Authors: Kotian, S;Carnes, RM;Stern, JL;
Genes
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Cholesterol depletion decreases adhesion of non-small cell lung cancer (NSCLC) cells to E-selectin
Authors: Amina Mohammadalipour, Christian A. Showalter, Harrison T. Muturi, Amir M. Farnoud, Sonia M. Najjar, Monica M. Burdick
American Journal of Physiology-Cell Physiology
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Protein corona investigations of polyplexes with varying hydrophobicity - From method development to in vitro studies
Authors: Hartl, N;Jürgens, DC;Carneiro, S;König, AC;Xiao, X;Liu, R;Hauck, SM;Merkel, OM;
International journal of pharmaceutics
Species: Human
Sample Types: Whole Cells
Applications: Cell Culture -
Negative regulation of CD44st by miR-138-5p affects the invasive ability of breast cancer cells and patient prognosis after breast cancer surgery
Authors: FX Jian, PX Bao, WF Li, YH Cui, HG Hong
BMC Cancer, 2023-03-24;23(1):269.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Novel Antibody Exerts Antitumor Effect through Downregulation of CD147 and Activation of Multiple Stress Signals
Authors: Keisuke Fukuchi, Kayoko Nanai, Hiroshi Yuita, Chikako Maru, Jun Tsukada, Masato Ishigami et al.
Journal of Oncology
-
Hematopoietic progenitors polarize in contact with bone marrow stromal cells in response to SDF1
Authors: Thomas Bessy, Adrian Candelas, Benoit Souquet, Khansa Saadallah, Alexandre Schaeffer, Benoit Vianay et al.
Journal of Cell Biology
-
Establishment and characterization of a primary cell culture derived from external auditory canal squamous cell carcinoma
Authors: Yuki Sekino, Akira Imaizumi, Noritaka Komune, Mayumi Ono, Kuniaki Sato, Shogo Masuda et al.
FEBS Open Bio
-
Overexpression of CD44v8-10 in Colon Polyps—A Possible Key to Early Diagnosis
Authors: Milan Dastych, Frantisek Hubatka, Pavlina Turanek-Knotigova, Josef Masek, Radek Kroupa, Milan Raška et al.
Pathology and Oncology Research
Species: Human
Sample Types: Cell Lysates, Whole Tissue
Applications: Western Blot, Immunocytochemistry -
Role of A Novel Angiogenesis FKBPL-CD44 Pathway in Preeclampsia Risk Stratification and Mesenchymal Stem Cell Treatment
Authors: Naomi Todd, Ross McNally, Abdelrahim Alqudah, Djurdja Jerotic, Sonja Suvakov, Danilo Obradovic et al.
The Journal of Clinical Endocrinology & Metabolism
-
RUNX2 interacts with BRG1 to target CD44 for promoting invasion and migration of colorectal cancer cells
Authors: X Yan, D Han, Z Chen, C Han, W Dong, L Han, L Zou, J Zhang, Y Liu, J Chai
Cancer Cell Int, 2020-10-15;20(0):505.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Essential Functions of Glycans in Human Epithelia Dissected by a CRISPR-Cas9-Engineered Human Organotypic Skin Model
Authors: Sally Dabelsteen, Emil M.H. Pallesen, Irina N. Marinova, Mathias I. Nielsen, Maria Adamopoulou, Troels B. Rømer et al.
Developmental Cell
-
Exosomal 2′,3′-CNP from mesenchymal stem cells promotes hippocampus CA1 neurogenesis/neuritogenesis and contributes to rescue of cognition/learning deficiencies of damaged brain
Authors: Shih-Yin Chen, Meng-chieh Lin, Jia-Shiuan Tsai, Pei-Lin He, Wen-Ting Luo, Ing-Ming Chiu et al.
Stem Cells Translational Medicine
-
HDAC7 regulates histone 3 lysine 27 acetylation and transcriptional activity at super-enhancer-associated genes in breast cancer stem cells
Authors: Corrado Caslini, Sunhwa Hong, Yuguang J. Ban, Xi S. Chen, Tan A. Ince
Oncogene
-
Toll‑like receptor 4 plays a tumor‑suppressive role in cutaneous squamous cell carcinoma
Authors: Erina Mikami, Mitsuhiro Kudo, Ryuji Ohashi, Kiyoko Kawahara, Yoko Kawamoto, Kiyoshi Teduka et al.
International Journal of Oncology
-
Fluoxetine induces direct inhibitory effects on mesenchymal stem cell?derived osteoprogenitor cells independent of serotonin concentration
Authors: SM Koura, M Salama, M El-Hussiny, MEA Khalil, A Lotfy, SA Hassan, SA Gad Elhak, MA Sobh
Mol Med Rep, 2019-02-01;0(0):.
Species: Rat
Sample Types: Whole Cells
Applications: Flow Cytometry -
The deubiquitylase OTUB1 mediates ferroptosis via stabilization of SLC7A11
Authors: T Liu, L Jiang, O Tavana, W Gu
Cancer Res., 2019-02-01;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Western Blot -
Secondary lymphoid organ fibroblastic reticular cells mediate trans-infection of HIV-1 via CD44-hyaluronan interactions
Authors: T Murakami, J Kim, Y Li, GE Green, A Shikanov, A Ono
Nat Commun, 2018-06-22;9(1):2436.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Optimizing human Treg immunotherapy by Treg subset selection and E-selectin ligand expression
Authors: C Donnelly, B Dykstra, N Mondal, J Huang, BJ Kaskow, R Griffin, R Sackstein, C Baecher-Al
Sci Rep, 2018-01-11;8(1):420.
Species: Human
Sample Types: Cell Lysates
Applications: Immunoprecipitation -
The CD44 standard isoform contributes to radioresistance of pancreatic cancer cells
Authors: Kento Tsubouchi, Kazumasa Minami, Naoki Hayashi, Yuhki Yokoyama, Seiji Mori, Hirofumi Yamamoto et al.
Journal of Radiation Research
-
MUC1 O-glycosylation contributes to anoikis resistance in epithelial cancer cells
Authors: Tushar Piyush, Jonathan M Rhodes, Lu-Gang Yu
Cell Death Discovery
-
Akt signaling is sustained by a CD44 splice isoform-mediated positive feedback loop
Authors: S Liu, C Cheng
Cancer Res., 2017-05-22;0(0):.
Species: Human
Sample Types: Cell Lysates, Whole Cells
Applications: Neutralization, Western Blot -
Cell-Specific Variation in E-Selectin Ligand Expression among Human Peripheral Blood Mononuclear Cells: Implications for Immunosurveillance and Pathobiology
Authors: M Silva, RK Fung, CB Donnelly, PA Videira, R Sackstein
J. Immunol, 2017-03-22;0(0):.
Species: Human
Sample Types: Cell Lysates, Whole Cells
Applications: Flow Cytometry, Immunoprecipitation, Western Blot -
Glycoengineering of E-selectin ligands by intracellular versus extracellular fucosylation differentially affects osteotropism of human mesenchymal stem cells
Authors: Brad Dykstra
Stem Cells, 2016-07-17;0(0):.
Species: Human
Sample Types: Cell Lysates
Applications: Immunoprecipitation -
PiggyBac transposon-mediated gene delivery efficiently generates stable transfectants derived from cultured primary human deciduous tooth dental pulp cells (HDDPCs) and HDDPC-derived iPS cells.
Authors: Inada E, Saitoh I, Watanabe S, Aoki R, Miura H, Ohtsuka M, Murakami T, Sawami T, Yamasaki Y, Sato M
Int J Oral Sci, 2015-09-14;7(3):144-54.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Quantitative Characterization of E-selectin Interaction with Native CD44 and P-selectin Glycoprotein Ligand-1 (PSGL-1) Using a Real Time Immunoprecipitation-based Binding Assay.
Authors: AbuSamra D, Al-Kilani A, Hamdan S, Sakashita K, Gadhoum S, Merzaban J
J Biol Chem, 2015-06-29;290(35):21213-30.
Species: Human
Sample Types: Cell Lysates, Protein
Applications: Surface Plasmon Resonance, Western Blot -
C6-ceramide nanoliposome suppresses tumor metastasis by eliciting PI3K and PKCzeta tumor-suppressive activities and regulating integrin affinity modulation.
Authors: Zhang P, Fu C, Hu Y, Dong C, Song Y, Song E
Sci Rep, 2015-03-20;5(0):9275.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
MUC1 extracellular domain confers resistance of epithelial cancer cells to anoikis
Authors: Q Zhao, T Piyush, C Chen, M A Hollingsworth, J Hilkens, J M Rhodes et al.
Cell Death & Disease
-
Accumulation of Extracellular Hyaluronan by Hyaluronan Synthase 3 Promotes Tumor Growth and Modulates the Pancreatic Cancer Microenvironment
Authors: Anne Kultti, Chunmei Zhao, Netai C. Singha, Susan Zimmerman, Ryan J. Osgood, Rebecca Symons et al.
BioMed Research International
-
MIF inhibits monocytic movement through a non-canonical receptor and disruption of temporal Rho GTPase activities in U-937 cells.
Authors: DiCosmo-Ponticello C, Hoover D, Coffman F, Cohen S, Cohen M
Cytokine, 2014-06-06;69(1):47-55.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Coexpression of EpCAM, CD44 variant isoforms and claudin-7 in anaplastic thyroid carcinoma.
Authors: Okada, Toshihir, Nakamura, Teruo, Watanabe, Takayuki, Onoda, Naoyoshi, Ashida, Atsuko, Okuyama, Ryuhei, Ito, Ken-ichi
PLoS ONE, 2014-04-11;9(4):e94487.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
CD26 expression on T-anaplastic large cell lymphoma (ALCL) line Karpas 299 is associated with increased expression of versican and MT1-MMP and enhanced adhesion.
Authors: Havre, Pamela A, Dang, Long H, Ohnuma, Kei, Iwata, Satoshi, Morimoto, Chikao, Dang, Nam H
BMC Cancer, 2013-11-01;13(0):517.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
A hexadecylamide derivative of hyaluronan (HYMOVIS®) has superior beneficial effects on human osteoarthritic chondrocytes and synoviocytes than unmodified hyaluronan
Authors: Margaret M Smith, Amy K Russell, Antonella Schiavinato, Christopher B Little
Journal of Inflammation
-
High molecular weight hyaluronan mediates the cancer resistance of the naked mole-rat
Authors: Xiao Tian, Jorge Azpurua, Christopher Hine, Amita Vaidya, Max Myakishev-Rempel, Julia Ablaeva et al.
Nature
-
The Anti-Migratory Effects of FKBPL and Its Peptide Derivative, AD-01: Regulation of CD44 and the Cytoskeletal Pathway
Authors: Anita Yakkundi, Lynn McCallum, Anthony O’Kane, Hayder Dyer, Jenny Worthington, Hayley D. McKeen et al.
PLoS ONE
-
HER2-associated radiation resistance of breast cancer stem cells isolated from HER2-negative breast cancer cells
Authors: Nadire Duru, Ming Fan, Demet Candas, Cheikh Menaa, Hsin-Chen Liu, Danupon Nantajit et al.
Clinical Cancer Research
Species: Human
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Expression of cancer stem cell markers in pancreatic intraepithelial neoplasias and pancreatic ductal adenocarcinomas
Authors: SHOKO KURE, YOKO MATSUDA, MASAHITO HAGIO, JUNJI UEDA, ZENYA NAITO, TOSHIYUKI ISHIWATA
International Journal of Oncology
-
KCa3.1 and TRPM7 channels at the uropod regulate migration of activated human T cells.
Authors: Kuras Z, Yun Y, Chimote A, Neumeier L, Conforti L
PLoS ONE, 2012-08-27;7(8):e43859.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Loss of desmocollin-2 confers a tumorigenic phenotype to colonic epithelial cells through activation of Akt/ beta -catenin signaling
Authors: Keli Kolegraff, Porfirio Nava, My N. Helms, Charles A. Parkos, Asma Nusrat
Molecular Biology of the Cell
-
Increased NOS2 predicts poor survival in estrogen receptor-negative breast cancer patients.
Authors: Glynn SA, Boersma BJ, Dorsey TH, Yi M, Yfantis HG, Ridnour LA, Martin DN, Switzer CH, Hudson RS, Wink DA, Lee DH, Stephens RM, Ambs S
J. Clin. Invest., 2010-10-18;120(11):3843-54.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Synovial sarcoma is a stem cell malignancy.
Authors: Naka N, Takenaka S, Araki N
Stem Cells, 2010-07-01;28(7):1119-31.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Anticancer chemotherapy inhibits MHC class I-related chain a ectodomain shedding by downregulating ADAM10 expression in hepatocellular carcinoma.
Authors: Kohga K, Takehara T, Tatsumi T, Miyagi T, Ishida H, Ohkawa K, Kanto T, Hiramatsu N, Hayashi N
Cancer Res., 2009-10-13;69(20):8050-7.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
The E-selectin ligand basigin/CD147 is responsible for neutrophil recruitment in renal ischemia/reperfusion.
Authors: Kato N, Yuzawa Y, Kosugi T
J. Am. Soc. Nephrol., 2009-05-14;20(7):1565-76.
Species: Mouse
Sample Types: Tissue Homogenates
Applications: Western Blot -
Inflammatory cytokines stimulate the adhesion of colon carcinoma cells to mesothelial monolayers.
Authors: van Grevenstein WM, Hofland LJ, van Rossen ME, van Koetsveld PM, Jeekel J, van Eijck CH
Dig. Dis. Sci., 2007-03-30;52(10):2775-83.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Cortactin underpins CD44-promoted invasion and adhesion of breast cancer cells to bone marrow endothelial cells.
Authors: Hill A, McFarlane S, Mulligan K, Gillespie H, Draffin JE, Trimble A, Ouhtit A, Johnston PG, Harkin DP, McCormick D, Waugh DJ
Oncogene, 2006-05-01;25(45):6079-91.
Species: Human
Sample Types: Cell Lysates, Whole Cells
Applications: ICC, Western Blot -
CD44 potentiates the adherence of metastatic prostate and breast cancer cells to bone marrow endothelial cells.
Authors: Draffin JE, McFarlane S, Hill A, Johnston PG, Waugh DJ
Cancer Res., 2004-08-15;64(16):5702-11.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry, ICC -
A novel ZEB1/HAS2 positive feedback loop promotes EMT in breast cancer
Authors: BT Preca, K Bajdak, K Mock, W Lehmann, V Sundararaj, P Bronsert, A Matzge-Ogi, V Orian-Rous, S Brabletz, T Brabletz, J Maurer, MP Stemmler
Oncotarget, 2017-02-14;0(0):.
-
Accessibilome of Human Glioblastoma: Collagen-VI-alpha-1 Is a new Target and a Marker of Poor Outcome.
Authors: Turtoi A, Blomme A, Bianchi E et al.
J. Proteome Res.
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Human CD44s Pan Specific Antibody
Average Rating: 4.7 (Based on 9 Reviews)
Have you used Human CD44s Pan Specific Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by: