Human CCL20/MIP-3 alpha DuoSet ELISA Summary
* Provided that the recommended microplates, buffers, diluents, substrates and solutions are used, and the assay is run as summarized in the Assay Procedure provided.
This DuoSet ELISA Development kit contains the basic components required for the development of sandwich ELISAs to measure natural and recombinant human CCL20/MIP-3alpha. The suggested diluent is suitable for the analysis of most cell culture supernate samples. Diluents for complex matrices, such as serum and plasma, should be evaluated prior to use in this DuoSet.
Product Features
- Optimized capture and detection antibody pairings with recommended concentrations save lengthy development time
- Development protocols are provided to guide further assay optimization
- Assay can be customized to your specific needs
- Economical alternative to complete kits
Kit Content
- Capture Antibody
- Detection Antibody
- Recombinant Standard
- Streptavidin conjugated to horseradish-peroxidase (Streptavidin-HRP)
Other Reagents Required
DuoSet Ancillary Reagent Kit 2 (5 plates): (Catalog # DY008) containing 96 well microplates, plate sealers, substrate solution, stop solution, plate coating buffer (PBS), wash buffer, and Reagent Diluent Concentrate 2.
The components listed above may be purchased separately:
PBS: (Catalog # DY006), or 137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4, pH 7.2 - 7.4, 0.2 µm filtered
Wash Buffer: (Catalog # WA126), or 0.05% Tween® 20 in PBS, pH 7.2-7.4
Reagent Diluent: (Catalog # DY995), or 1% BSA in PBS, pH 7.2-7.4, 0.2 µm filtered
Substrate Solution: 1:1 mixture of Color Reagent A (H2O2) and Color Reagent B (Tetramethylbenzidine) (Catalog # DY999)
Stop Solution: 2 N H2SO4 (Catalog # DY994)
Microplates: R&D Systems (Catalog # DY990)
Plate Sealers: ELISA Plate Sealers (Catalog # DY992)
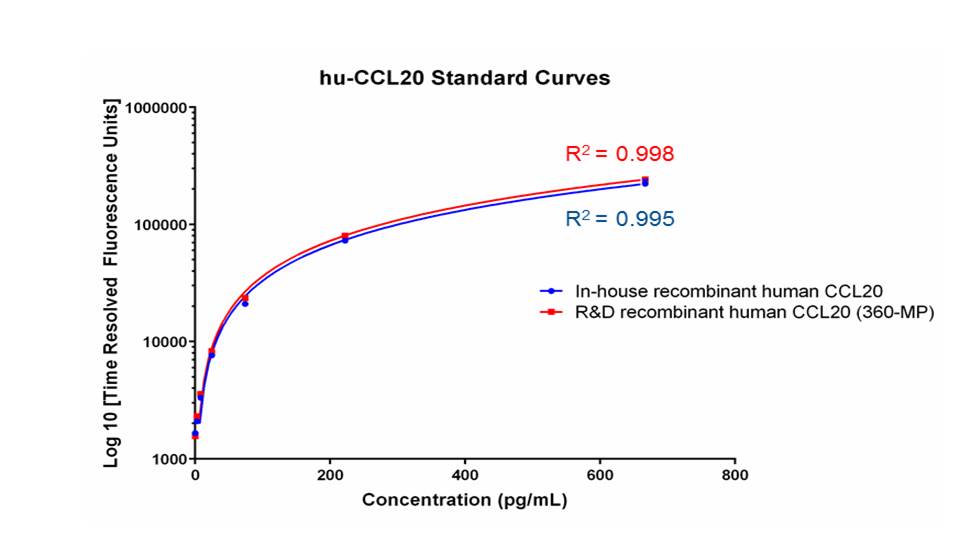
Scientific Data
Product Datasheets
Preparation and Storage
Background: CCL20/MIP-3 alpha
CCL20, also known as MIP-3 alpha, is a chemokine that plays an important role in dendritic cell trafficking and recruitment and activation of T cells. It is upregulated in monocytes, T cells, endothelial cells, epithelial cells, and fibroblasts following inflammatory stimulation. CCL20 signals through CCR6 which is expressed on memory T cells, B cells and bone marrow-derived dendritic cells.
Assay Procedure
GENERAL ELISA PROTOCOL
Plate Preparation
- Dilute the Capture Antibody to the working concentration in PBS without carrier protein. Immediately coat a 96-well microplate with 100 μL per well of the diluted Capture Antibody. Seal the plate and incubate overnight at room temperature.
- Aspirate each well and wash with Wash Buffer, repeating the process two times for a total of three washes. Wash by filling each well with Wash Buffer (400 μL) using a squirt bottle, manifold dispenser, or autowasher. Complete removal of liquid at each step is essential for good performance. After the last wash, remove any remaining Wash Buffer by aspirating or by inverting the plate and blotting it against clean paper towels.
- Block plates by adding 300 μL Reagent Diluent to each well. Incubate at room temperature for a minimum of 1 hour.
- Repeat the aspiration/wash as in step 2. The plates are now ready for sample addition.
Assay Procedure
- Add 100 μL of sample or standards in Reagent Diluent, or an appropriate diluent, per well. Cover with an adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the Detection Antibody, diluted in Reagent Diluent, to each well. Cover with a new adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the working dilution of Streptavidin-HRP to each well. Cover the plate and incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Repeat the aspiration/wash as in step 2.
- Add 100 μL of Substrate Solution to each well. Incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Add 50 μL of Stop Solution to each well. Gently tap the plate to ensure thorough mixing.
- Determine the optical density of each well immediately, using a microplate reader set to 450 nm. If wavelength correction is available, set to 540 nm or 570 nm. If wavelength correction is not available, subtract readings at 540 nm or 570 nm from the readings at 450 nm. This subtraction will correct for optical imperfections in the plate. Readings made directly at 450 nm without correction may be higher and less accurate.
Citations for Human CCL20/MIP-3 alpha DuoSet ELISA
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
39
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Pro-Inflammatory Properties of Salivary Gland-Derived Fibroblasts-Implications in Sjögren's Disease
Authors: Brunner, M;Guggisberg, D;Sprecher, M;Pastva, O;Bürki, K;Houtman, M;Kreuzer, M;Krättli, SA;Jahnke, L;Roceri, M;Bruggmann, R;Elhai, M;Maurer, B;Marti, TM;Ospelt, C;Klein, K;
Cells
Species: Human
Sample Types: Cell Culture Supernates
-
Effect of progestin-based contraceptives on HIV-associated vaginal immune biomarkers and microbiome in adolescent girls
Authors: Nasr, MA;Aldous, A;Daniels, J;Joy, C;Capozzi, E;Yang, M;Moriarty, P;Emmanuel-Baker, V;Malcolm, S;Green, SJ;Gomez-Lobo, V;Ghosh, M;
PloS one
Species: Human
Sample Types: Vaginal Swab
-
Using the Traditional Ex Vivo Whole Blood Model to Discriminate Bacteria by Their Inducible Host Responses
Authors: Chick, HM;Rees, ME;Lewis, ML;Williams, LK;Bodger, O;Harris, LG;Rushton, S;Wilkinson, TS;
Biomedicines
Species: Human
Sample Types: Whole Blood
-
The Endogenous Dual Retinoid Receptor Agonist Alitretinoin Exhibits Immunoregulatory Functions on Antigen-Presenting Cells
Authors: Kislat, A;Olah, P;Kuchner, M;Gerber, PA;Schrader, J;Meller, S;Homey, B;
International journal of molecular sciences
Species: Human
Sample Types: Cell Culture Supernates
-
Repeated exposure of bronchial epithelial cells to particular matter increases allergen-induced cytokine release and permeability
Authors: H Janbazacya, J van Bergen, S Varasteh, J Garssen, G Folkerts, S Braber
Cytokine, 2022-04-08;154(0):155878.
Species: Human
Sample Types: Cell Culture Supernates
-
Asthmatic bronchial smooth muscle increases rhinovirus replication within the bronchial epithelium
Authors: P Esteves, B Allard, A Celle, I Dupin, E Maurat, O Ousova, M Thumerel, JW Dupuy, T Leste-Lass, R Marthan, PO Girodet, T Trian, P Berger
Cell Reports, 2022-03-29;38(13):110571.
Species: Human
Sample Types: Cell Culture Supernates
-
Echinococcus multilocularis specific antibody, systemic cytokine, and chemokine levels, as well as antigen-specific cellular responses in patients with progressive, stable, and cured alveolar echinococcosis: A 10-year follow-up
Authors: B Grüner, L Peters, A Hillenbran, P Vo beta berg, J Schweiker, EG Rollmann, LH Rodriguez, J Blumhardt, S Burkert, P Kern, C Köhler, PT Soboslay
PloS Neglected Tropical Diseases, 2022-02-02;16(2):e0010099.
Species: Human
Sample Types: Cell Culture Supernates
-
Chemerin Overexpression in the Liver Protects against Inflammation in Experimental Non-Alcoholic Steatohepatitis
Authors: R Pohl, S Feder, EM Haberl, L Rein-Fisch, TS Weiss, M Spirk, A Bruckmann, N McMullen, CJ Sinal, C Buechler
Biomedicines, 2022-01-07;10(1):.
Species: Human
Sample Types: Cell Culture Supernates
-
DRP1-Mediated Mitochondrial Fission Regulates Lung Epithelial Response to Allergen
Authors: SR Bruno, A Kumar, ZF Mark, R Chandrasek, E Nakada, N Chamberlai, B Mihavics, J Walzer, J Cahoon, AE Dixon, B Cunniff, V Anathy
International Journal of Molecular Sciences, 2021-10-15;22(20):.
Species: Human
Sample Types: Cell Culture Supernates
-
Multi-level inhibition of coronavirus replication by chemical ER stress
Authors: MS Shaban, C Müller, C Mayr-Buro, H Weiser, J Meier-Soel, BV Albert, A Weber, U Linne, T Hain, I Babayev, N Karl, N Hofmann, S Becker, S Herold, ML Schmitz, J Ziebuhr, M Kracht
Nature Communications, 2021-09-20;12(1):5536.
Species: Human
Sample Types: Cell Culture Supernates
-
Amino terminal recognition by a CCR6 chemokine receptor antibody blocks CCL20 signaling and IL-17 expression via beta-arrestin
Authors: S Gómez-Mele, FI García-Mac, T García-Mac, V Luna-Guerr, G Montero-Pe, I Túnez-Fiña, E Paz-Rojas
BMC biotechnology, 2021-07-05;21(1):41.
Species: Human
Sample Types: Cell Culture Supernates
-
Patient Derived Colonoids as Drug Testing Platforms-Critical Importance of Oxygen Concentration
Authors: HK Skovdahl, S Gopalakris, TD Svendsen, AVB Granlund, I Bakke, ZG Ginbot, S Thorsvik, A Flatberg, B Sporsheim, J Ostrop, TE Mollnes, AK Sandvik, T Bruland
Frontiers in Pharmacology, 2021-05-13;12(0):679741.
Species: Human
Sample Types: Cell Culture Supernates
-
Adipocytokines in Untreated Newly Diagnosed Rheumatoid Arthritis: Association with Circulating Chemokines and Markers of Inflammation
Authors: GK Vasileiadi, AC Lundell, Y Zhang, K Andersson, I Gjertsson, A Rudin, C Maglio
Biomolecules, 2021-02-21;11(2):.
Species: Human
Sample Types: Plasma
-
Tissue-specific endothelial cell heterogeneity contributes to unequal inflammatory responses
Authors: H Gunawardan, T Romero, N Yao, S Heidt, A Mulder, DA Elashoff, NM Valenzuela
Scientific Reports, 2021-01-21;11(1):1949.
Species: Human
Sample Types: Cell Culture Supernates
-
Gene expression network analyses during infection with virulent and avirulent Trypanosoma cruzi�strains unveil a role for fibroblasts in neutrophil recruitment and activation
Authors: AER Oliveira, MCA Pereira, AT Belew, LRP Ferreira, LMN Pereira, EGA Neves, MDCP Nunes, BA Burleigh, WO Dutra, NM El-Sayed, RT Gazzinelli, SMR Teixeira
PLoS Pathog., 2020-08-18;16(8):e1008781.
Species: Human
Sample Types: Cell Culture Supernates
-
Intracellular virus sensor MDA5 exacerbates vitiligo by inducing the secretion of chemokines in keratinocytes under virus invasion
Authors: T Zhuang, X Yi, J Chen, P Kang, X Chen, J Chen, T Cui, Y Chang, Z Ye, Q Ni, Y Wang, P Du, B Li, L Liu, Z Jian, K Li, T Gao, S Li, C Li
Cell Death Dis, 2020-06-12;11(6):453.
Species: Human
Sample Types: Cell Culture Supernates
-
Colonic microbiota is associated with inflammation and host epigenomic alterations in inflammatory bowel disease
Authors: FJ Ryan, AM Ahern, RS Fitzgerald, EJ Laserna-Me, EM Power, AG Clooney, KW O'Donoghue, PJ McMurdie, S Iwai, A Crits-Chri, D Sheehan, C Moran, B Flemer, AL Zomer, A Fanning, J O'Callagha, J Walton, A Temko, W Stack, L Jackson, SA Joyce, S Melgar, TZ DeSantis, JT Bell, F Shanahan, MJ Claesson
Nat Commun, 2020-03-23;11(1):1512.
Species: Human
Sample Types: Cell Culture Supernates
-
Human BCL-G regulates secretion of inflammatory chemokines but is dispensable for induction of apoptosis by IFN-gamma and TNF-alpha in intestinal epithelial cells
Authors: JA Woznicki, P Flood, M Bustamante, P Stamou, G Moloney, A Fanning, SA Zulquernai, J McCarthy, F Shanahan, S Melgar, K Nally
Cell Death Dis, 2020-01-27;11(1):68.
Species: Human
Sample Types: Cell Culture Supernates
-
Tumor-derived CCL20 affects B16 melanoma growth in mice
Authors: D Martin-Gar, C Silva-Vilc, R Will, AH Enk, AS Lonsdorf
J. Dermatol. Sci., 2019-12-13;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
SIRT1/FoxO3 axis alteration leads to aberrant immune responses in bronchial epithelial cells
Authors: S Di Vincenz, IH Heijink, JA Noordhoek, C Cipollina, L Siena, A Bruno, M Ferraro, DS Postma, M Gjomarkaj, E Pace
J. Cell. Mol. Med., 2018-02-07;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Distinctive expression of T cell guiding molecules in human autoimmune lymph node stromal cells upon TLR3 triggering
Authors: JS Hähnlein, TH Ramwadhdoe, JF Semmelink, IY Choi, FH Berger, M Maas, DM Gerlag, PP Tak, TBH Geijtenbee, LGM van Baarse
Sci Rep, 2018-01-29;8(1):1736.
Species: Human
Sample Types: Cell Culture Supernates
-
Rheumatoid arthritis bone marrow environment supports Th17 response.
Authors: Ewa Kuca-Warna, Weronika Kurowska, Monika Prochorec, Anna Radzikows, Tomasz Burakowsk, Urszula Skalska, Magdalena Massalska, Magdalena Pleba?czy, Barbara Ma?dyk-No, Iwona S?owi?ska, Robert Gasik, W?odzimierz Ma?li?ski
Arthritis Research & Therapy, 2017-12-08;0(0):1478-6362.
Species: Human
Sample Types: Plasma
-
IL-27 Modulates Chemokine Production in TNF-? -Stimulated Human Oral Epithelial Cells
Authors: Y Hosokawa, I Hosokawa, K Ozaki, T Matsuo
Cell. Physiol. Biochem., 2017-10-05;43(3):1198-1206.
Species: Human
Sample Types: Cell Culture Supernates
-
Human Lymph Node-Derived Fibroblastic and Double-Negative Reticular Cells Alter Their Chemokines and Cytokines Expression Profile Following Inflammatory Stimuli
Authors: P Severino, DT Palomino, H Alvarenga, CB Almeida, DC Pasqualim, A Cury, PR Salvalaggi, AL De Vasconc, MC Andrade, T Aloia, S Bromberg, LV Rizzo, FA Rocha, LC Marti
Front Immunol, 2017-02-14;8(0):141.
Species: Human
Sample Types: Cell Culture Supernates
-
Transforming Growth Factor-beta and Interleukin-1beta Signaling Pathways Converge on the Chemokine CCL20 Promoter.
Authors: Brand O, Somanath S, Moermans C, Yanagisawa H, Hashimoto M, Cambier S, Markovics J, Bondesson A, Hill A, Jablons D, Wolters P, Lou J, Marks J, Baron J, Nishimura S
J Biol Chem, 2015-04-27;290(23):14717-28.
Species: Human
Sample Types: Tissue Homogenates
-
Bacterial siderophores that evade or overwhelm lipocalin 2 induce hypoxia inducible factor 1alpha and proinflammatory cytokine secretion in cultured respiratory epithelial cells.
Authors: Holden V, Lenio S, Kuick R, Ramakrishnan S, Shah Y, Bachman M
Infect Immun, 2014-06-30;82(9):3826-36.
Species: Human
Sample Types: Cell Culture Supernates
-
Selective activation of human dendritic cells by OM-85 through a NF-kB and MAPK dependent pathway.
Authors: Parola C, Salogni L, Vaira X, Scutera S, Somma P, Salvi V, Musso T, Tabbia G, Bardessono M, Pasquali C, Mantovani A, Sozzani S, Bosisio D
PLoS ONE, 2013-12-30;8(12):e82867.
Species: Human
Sample Types: Cell Culture Supernates
-
A multiplexed assay to detect antimicrobial peptides in biological fluids and cell secretions
Authors: Austin W. Boesch, Yifeng Zhao, Alison S. Landman, Marta Rodriguez Garcia, John V. Fahey, Charles R. Wira et al.
Journal of Immunological Methods
Species: Human
Sample Types: Vaginal Lavage Fluid
-
Human Papillomavirus Type 8 Interferes with a Novel C/EBPβ-Mediated Mechanism of Keratinocyte CCL20 Chemokine Expression and Langerhans Cell Migration.
Authors: Sperling T, Oldak M, Walch-Ruckheim B, Wickenhauser C, Doorbar J, Pfister H, Malejczyk M, Majewski S, Keates AC, Smola S
PLoS Pathog., 2012-07-26;8(7):e1002833.
Species: Human
Sample Types: Cell Culture Supernates
-
Candida albicans yeast and hyphae are discriminated by MAPK signaling in vaginal epithelial cells.
Authors: Moyes DL, Murciano C, Runglall M, Islam A, Thavaraj S, Naglik JR
PLoS ONE, 2011-11-08;6(11):e26580.
Species: Human
Sample Types: Cell Culture Supernates
-
Identification of a New Phenotype of Tolerogenic Human Dendritic Cells Induced by Fungal Proteases from Aspergillus oryzae.
Authors: Zimmer A, Luce S, Gaignier F, Nony E, Naveau M, Biola-Vidamment A, Pallardy M, Van Overtvelt L, Mascarell L, Moingeon P
J. Immunol., 2011-03-02;186(7):3966-76.
Species: Human
Sample Types: Cell Culture Supernates
-
CCL20/CCR6 feedback exaggerates epidermal growth factor receptor-dependent MUC5AC mucin production in human airway epithelial (NCI-H292) cells.
Authors: Kim S, Lewis C, Nadel JA
J. Immunol., 2011-02-07;186(6):3392-400.
Species: Human
Sample Types: Cell Lysates
-
IL-17 and IL-22 mediate IL-20 subfamily cytokine production in cultured keratinocytes via increased IL-22 receptor expression.
Authors: Tohyama M, Hanakawa Y, Shirakata Y, Dai X, Yang L, Hirakawa S, Tokumaru S, Okazaki H, Sayama K, Hashimoto K
Eur. J. Immunol., 2009-10-01;39(10):2779-88.
Species: Human
Sample Types: Cell Culture Supernates
-
Interleukin-17 and interferon-gamma are produced concomitantly by human coronary artery-infiltrating T cells and act synergistically on vascular smooth muscle cells.
Authors: Eid RE, Rao DA, Zhou J, Lo SF, Ranjbaran H, Gallo A, Sokol SI, Pfau S, Pober JS, Tellides G
Circulation, 2009-03-02;119(10):1424-32.
Species: Human
Sample Types: Plasma
-
The role of a nuclear protein, histone H1, on signalling pathways for the maturation of dendritic cells.
Authors: Hsu LW, Chen CL, Nakano T, Lai CY, Chiang KC, Lin YC, Kao YH, Chen SH, Goto T, Sung WC, Yang CH, Cheng YF, Jawan B, Chiu KW, Goto S
Clin. Exp. Immunol., 2008-04-24;152(3):576-84.
Species: Human
Sample Types: BALF
-
Lactoferrin, a major defense protein of innate immunity, is a novel maturation factor for human dendritic cells.
Authors: Spadaro M, Caorsi C, Ceruti P, Varadhachary A, Forni G, Pericle F, Giovarelli M
FASEB J., 2008-03-25;22(8):2747-57.
Species: Human
Sample Types: Cell Culture Supernates
-
Lipopolysaccharide-squamous cell carcinoma-monocyte interactions induce cancer-supporting factors leading to rapid STAT3 activation.
Authors: Kurago ZB, Lam-ubol A, Stetsenko A
Head Neck Pathol, 2008-03-01;2(1):1-12.
Species: Human
Sample Types: Cell Culture Supernates
-
The effects of IL-20 subfamily cytokines on reconstituted human epidermis suggest potential roles in cutaneous innate defense and pathogenic adaptive immunity in psoriasis.
Authors: Sa SM, Valdez PA, Wu J
J. Immunol., 2007-02-15;178(4):2229-40.
Species: Human
Sample Types: Cell Culture Supernates
-
Multiple NF-kappaB and IFN regulatory factor family transcription factors regulate CCL19 gene expression in human monocyte-derived dendritic cells.
Authors: Pietila TE, Veckman V, Lehtonen A, Lin R, Hiscott J, Julkunen I
J. Immunol., 2007-01-01;178(1):253-61.
Species: Human
Sample Types: Cell Culture Supernates
FAQs
No product specific FAQs exist for this product, however you may
View all ELISA FAQsReviews for Human CCL20/MIP-3 alpha DuoSet ELISA
Average Rating: 5 (Based on 2 Reviews)
Have you used Human CCL20/MIP-3 alpha DuoSet ELISA?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by: